Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones
- PMID: 15051872
- PMCID: PMC387387
- DOI: 10.1073/pnas.0306141101
Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones
Abstract
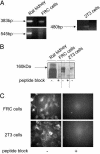
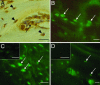
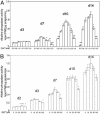
We investigated the direct effects of changes in free ionized extracellular calcium concentrations ([Ca2+]o) on osteoblast function and the involvement of the calcium-sensing receptor (CaR) in mediating these responses. CaR mRNA and protein were detected in osteoblast models, freshly isolated fetal rat calvarial cells and murine clonal osteoblastic 2T3 cells, and in freshly frozen, undecalcified preparations of human mandible and rat femur. In fetal rat calvarial cells, elevating [Ca2+]o and treatment with gadolinium, a nonpermeant CaR agonist, resulted in phosphorylation of the extracellular signal-regulated kinases 1 and 2, Akt, and glycogensynthase kinase 3beta, consistent with signals of cell survival and proliferation. In agreement, cell number was increased under these conditions. Expression of the osteoblast differentiation markers core binding factor alpha1, osteocalcin, osteopontin, and collagen I mRNAs was increased by high [Ca2+]o, as was mineralized nodule formation. Alkaline phosphatase activity was maximal for [Ca2+]o between 1.2 and 1.8 mM. Inhibition of CaR by NPS 89636 blocked responses to the CaR agonists. In conclusion, we show that small deviations of [Ca2+]o from physiological values have a profound impact on bone cell fate, by means of the CaR and independently of systemic calciotropic peptides.
Figures



References
-
- Parfitt, A. M. (1987) Bone 8, Suppl. 1, S1-S8. - PubMed
-
- Godwin, S. L. & Soltoff, S. P. (1997) J. Biol. Chem. 272, 11307-11312. - PubMed
-
- Yamaguchi, T., Chattopadhyay, N., Kifor, O., Butters, R. R., Jr., Sugimoto, T. & Brown, E. M. (1998) J. Bone Miner. Res. 13, 1530-1538. - PubMed
-
- Eklou-Kalonji, E., Denis, I., Lieberherr, M. & Pointillart, A. (1998) Cell Tissue Res. 292, 163-171. - PubMed
-
- Nakade, O., Takahashi, K., Takuma, T., Aoki, T. & Kaku, T. (2001) J. Bone Miner. Metab. 19, 13-19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

