The distribution and morphological characteristics of catecholaminergic cells in the diencephalon and midbrain of the bottlenose dolphin (Tursiops truncatus)
- PMID: 15051966
- PMCID: PMC8770345
- DOI: 10.1159/000077542
The distribution and morphological characteristics of catecholaminergic cells in the diencephalon and midbrain of the bottlenose dolphin (Tursiops truncatus)
Abstract
The present study describes the distribution and cellular morphology of catecholaminergic neurons in the diencephalon and midbrain of the bottlenose dolphin (Tursiops truncatus). Tyrosine hydroxylase immunohistochemistry was used to visualize these putatively dopaminergic neurons. The standard A1-A17, C1-C3, nomenclature is used for expediency; however, the neuroanatomical names of the various nuclei have also been given. Dolphins exhibit certain tyrosine hydroxylase immunoreactive (TH-ir) catecholaminergic neuronal groups in the midbrain (A8, A9, A10) and diencephalon (A11, A12, A14), however, no neuronal clusters clearly corresponding to the A13 and A15 groups could be identified. The subdivisions of these neuronal groups are in general agreement with those of other mammals, but there is a high degree of species specificity. First, three TH-ir neuronal groups not identified in other species were found: in the ventral lateral peri-aqueductal gray matter, posterior dorsal hypothalamus, and rostral mesencephalic raphe. Second, the normal components of the substantia nigra (A9 or pars compacta, A9 lateral or pars lateralis, A9 ventral or pars reticulata) were extremely cell sparse, but there was a substantial expansion of the A9 medial and A10 lateral subdivisions forming an impressive 'ventral wing' in the posterior substantia nigra. The findings of this and previous studies suggest a distinct evolutionary trend occurring in the neuromodulatory systems in mammals. The results are discussed in relation to motor control, thermoregulation, unihemispheric sleep, and dolphin cognition.
Copyright 2004 S. Karger AG, Basel
Figures



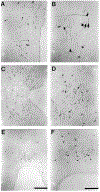
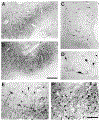


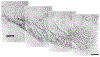

Similar articles
-
The distribution and morphological characteristics of catecholaminergic cells in the brain of monotremes as revealed by tyrosine hydroxylase immunohistochemistry.Brain Behav Evol. 2002;60(5):298-314. doi: 10.1159/000067193. Brain Behav Evol. 2002. PMID: 12476055
-
Differential expression of AMPA receptor subunits in dopamine neurons of the rat brain: a double immunocytochemical study.Neuroscience. 2001;106(1):149-60. doi: 10.1016/s0306-4522(01)00255-x. Neuroscience. 2001. PMID: 11564425
-
Immunohistochemical localization of serotoninergic, enkephalinergic, and catecholaminergic cells in the brainstem and diencephalon of a cartilaginous fish, Hydrolagus colliei.J Comp Neurol. 1991 Jul 22;309(4):535-48. doi: 10.1002/cne.903090409. J Comp Neurol. 1991. PMID: 1918446
-
Distribution of central catecholaminergic neurons: a comparison between ungulates, humans and other species.Histol Histopathol. 1998 Oct;13(4):1163-77. doi: 10.14670/HH-13.1163. Histol Histopathol. 1998. PMID: 9810508 Review.
-
The role of otx2 in adult mesencephalic-diencephalic dopaminergic neurons.Mol Neurobiol. 2011 Apr;43(2):107-13. doi: 10.1007/s12035-010-8148-y. Epub 2010 Nov 18. Mol Neurobiol. 2011. PMID: 21086067 Review.
Cited by
-
Dopamine: Functions, Signaling, and Association with Neurological Diseases.Cell Mol Neurobiol. 2019 Jan;39(1):31-59. doi: 10.1007/s10571-018-0632-3. Epub 2018 Nov 16. Cell Mol Neurobiol. 2019. PMID: 30446950 Free PMC article. Review.
-
Toothed Whales Have Black Neurons in the Blue Spot.Vet Sci. 2022 Sep 26;9(10):525. doi: 10.3390/vetsci9100525. Vet Sci. 2022. PMID: 36288139 Free PMC article.
-
Cetacean sleep: an unusual form of mammalian sleep.Neurosci Biobehav Rev. 2008 Oct;32(8):1451-84. doi: 10.1016/j.neubiorev.2008.05.023. Epub 2008 May 24. Neurosci Biobehav Rev. 2008. PMID: 18602158 Free PMC article. Review.
-
Timing and context of dolphin clicks during and after mine simulator detection and marking in the open ocean.Biol Open. 2018 Feb 20;7(2):bio031625. doi: 10.1242/bio.031625. Biol Open. 2018. PMID: 29463515 Free PMC article.
-
Neuroanatomy of the Cetacean Sensory Systems.Animals (Basel). 2023 Dec 23;14(1):66. doi: 10.3390/ani14010066. Animals (Basel). 2023. PMID: 38200796 Free PMC article. Review.
References
-
- Andén N-E, Carlsson A, Dahlström A, Fuxe K (1964) Demonstration and mapping out of nigro-neostriatal dopamine neurons. Life Sci 3: 523–530. - PubMed
-
- Andén N-E, Dahlström A, Fuxe K, Larsson K, Olson L, Ungerstedt U (1966) Ascending monoamine neurons to the telencephalon and diencephalon. Acta Physiol Scand 67:313–326.
-
- Battista A, Fuxe K, Goldstein M, Ogawa M (1972) Mapping of central monoamine neurons in the monkey. Experientia 28:688–690. - PubMed
-
- Björklund A, Lindvall O (1984) Dopamine-containing systems in the CNS. In: Handbook of Chemical Neuroanatomy, Vol. 2: Classical Transmitters in the CNS, Part 1. (Björklund A, Hökfelt T, eds), pp 55–122. Amsterdam: Elsevier.
-
- Björklund A, Skagerberg G (1979) Evidence for a major spinal cord projection from the diencephalic A11 dopamine cell group in the rat using transmitter-specific fluorescent retrograde tracing. Brain Res 177:170–175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

