Quantitative vibrational dynamics of iron in nitrosyl porphyrins
- PMID: 15053610
- PMCID: PMC1570756
- DOI: 10.1021/ja038526h
Quantitative vibrational dynamics of iron in nitrosyl porphyrins
Abstract
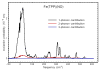
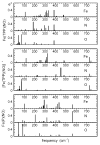
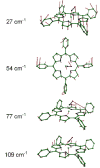
We use quantitative experimental and theoretical approaches to characterize the vibrational dynamics of the Fe atom in porphyrins designed to model heme protein active sites. Nuclear resonance vibrational spectroscopy (NRVS) yields frequencies, amplitudes, and directions for 57Fe vibrations in a series of ferrous nitrosyl porphyrins, which provide a benchmark for evaluation of quantum chemical vibrational calculations. Detailed normal mode predictions result from DFT calculations on ferrous nitrosyl tetraphenylporphyrin Fe(TPP)(NO), its cation [Fe(TPP)(NO)]+, and ferrous nitrosyl porphine Fe(P)(NO). Differing functionals lead to significant variability in the predicted Fe-NO bond length and frequency for Fe(TPP)(NO). Otherwise, quantitative comparison of calculated and measured Fe dynamics on an absolute scale reveals good overall agreement, suggesting that DFT calculations provide a reliable guide to the character of observed Fe vibrational modes. These include a series of modes involving Fe motion in the plane of the porphyrin, which are rarely identified using infrared and Raman spectroscopies. The NO binding geometry breaks the four-fold symmetry of the Fe environment, and the resulting frequency splittings of the in-plane modes predicted for Fe(TPP)(NO) agree with observations. In contrast to expectations of a simple three-body model, mode energy remains localized on the FeNO fragment for only two modes, an N-O stretch and a mode with mixed Fe-NO stretch and FeNO bend character. Bending of the FeNO unit also contributes to several of the in-plane modes, but no primary FeNO bending mode is identified for Fe(TPP)(NO). Vibrations associated with hindered rotation of the NO and heme doming are predicted at low frequencies, where Fe motion perpendicular to the heme is identified experimentally at 73 and 128 cm-1. Identification of the latter two modes is a crucial first step toward quantifying the reactive energetics of Fe porphyrins and heme proteins.
Figures










Similar articles
-
Oriented single-crystal nuclear resonance vibrational spectroscopy of [Fe(TPP)(MI)(NO)]: quantitative assessment of the trans effect of NO.Inorg Chem. 2010 Aug 2;49(15):7197-215. doi: 10.1021/ic1010677. Inorg Chem. 2010. PMID: 20586416 Free PMC article.
-
Nuclear resonance vibrational spectroscopy applied to [Fe(OEP)(NO)]: the vibrational assignments of five-coordinate ferrous heme-nitrosyls and implications for electronic structure.Inorg Chem. 2010 May 3;49(9):4133-48. doi: 10.1021/ic902181e. Inorg Chem. 2010. PMID: 20345089 Free PMC article.
-
Direct determination of the complete set of iron normal modes in a porphyrin-imidazole model for carbonmonoxy-heme proteins: [Fe(TPP)(CO)(1-MeIm)].J Am Chem Soc. 2003 Jun 11;125(23):6927-36. doi: 10.1021/ja028219w. J Am Chem Soc. 2003. PMID: 12783545
-
Nuclear resonance vibrational spectroscopy--NRVS.J Inorg Biochem. 2005 Jan;99(1):60-71. doi: 10.1016/j.jinorgbio.2004.11.004. J Inorg Biochem. 2005. PMID: 15598492 Review.
-
What Can Be Learned from Nuclear Resonance Vibrational Spectroscopy: Vibrational Dynamics and Hemes.Chem Rev. 2017 Oct 11;117(19):12532-12563. doi: 10.1021/acs.chemrev.7b00295. Epub 2017 Sep 18. Chem Rev. 2017. PMID: 28921972 Free PMC article. Review.
Cited by
-
Nuclear resonance vibrational spectroscopy and electron paramagnetic resonance spectroscopy of 57Fe-enriched [FeFe] hydrogenase indicate stepwise assembly of the H-cluster.Biochemistry. 2013 Feb 5;52(5):818-26. doi: 10.1021/bi301336r. Epub 2013 Jan 24. Biochemistry. 2013. PMID: 23249091 Free PMC article.
-
Oriented single-crystal nuclear resonance vibrational spectroscopy of [Fe(TPP)(MI)(NO)]: quantitative assessment of the trans effect of NO.Inorg Chem. 2010 Aug 2;49(15):7197-215. doi: 10.1021/ic1010677. Inorg Chem. 2010. PMID: 20586416 Free PMC article.
-
Dynamics of the [4Fe-4S] cluster in Pyrococcus furiosus D14C ferredoxin via nuclear resonance vibrational and resonance Raman spectroscopies, force field simulations, and density functional theory calculations.Biochemistry. 2011 Jun 14;50(23):5220-35. doi: 10.1021/bi200046p. Epub 2011 May 18. Biochemistry. 2011. PMID: 21500788 Free PMC article.
-
125Te and 57Fe nuclear resonance vibrational spectroscopic characterization of intermediate spin state mixed-valent dimers.Nat Commun. 2025 Jul 25;16(1):6843. doi: 10.1038/s41467-025-62118-w. Nat Commun. 2025. PMID: 40715092 Free PMC article.
-
Vibrational Perturbation of the [FeFe] Hydrogenase H-Cluster Revealed by 13C2H-ADT Labeling.J Am Chem Soc. 2021 Jun 9;143(22):8237-8243. doi: 10.1021/jacs.1c02323. Epub 2021 May 27. J Am Chem Soc. 2021. PMID: 34043346 Free PMC article.
References
-
- Abbreviations: NRVS, nuclear resonance vibrational spectroscopy (note that the terms nuclear (resonant) inelastic X-ray scattering and phonon-assisted Mössbauer effect have also been used for this technique); DFT, density functional theory; NOS, nitric oxide synthase; sGC, soluble guanylate cyclase; TPP, tetraphenylporphyrin; P, porphine; OEP, octaethylporphyrin; PPIXDME, protoporphyrin IX dimethyl ester; DPIXDME, deuteroporphyrin IX dimethyl ester; MPIXDME, mesoporphyrin IX dimethyl ester; VDOS, vibrational density of states; Npyr, pyrrole nitrogen; Im, imidazole; Py, pyridine.
-
- Spiro TG, editor. Wiley-Interscience; New York: 1988. Biological Applications of Raman Spectroscopy.
-
- Mäntele W. Trends Biochem Sci. 1993;18:197–202. - PubMed
-
- Fayer, M. D., Ed.; Ultrafast Infrared and Raman Spectroscopy; Marcel-Dekker: New York, 2001.
-
- Barth A, Zscherp C. Q Rev Biophys. 2002;35:369–430. - PubMed
References
-
- Dodd RE, Robinson PL. Experimental Inorganic Chemistry. Elsevier; New York: 1957. p. 253.
-
- Adler AD, Longo FR, Finarelli JD, Goldmacher J, Assour J, Korsakoff L. J Org Chem. 1967;32:476.
-
- Landergren M, Baltzer L. Inorg Chem. 1990;29:556.
-
- Fleischer EB, Srivastava TS. J Am Chem Soc. 1969;91:2403.
-
- Hoffman AB, Collins DM, Day VW, Fleischer EB, Srivastava TS, Hoard JL. J Am Chem Soc. 1972;94:3620. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

