ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons
- PMID: 15054132
- PMCID: PMC379687
- DOI: 10.1101/lm.67804
ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons
Abstract
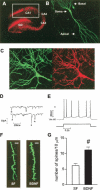
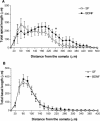
Brain-derived neurotrophic factor (BDNF) is a potent modulator of synaptic transmission and plasticity in the CNS, acting both pre- and postsynaptically. We demonstrated recently that BDNF/TrkB signaling increases dendritic spine density in hippocampal CA1 pyramidal neurons. Here, we tested whether activation of the prominent ERK (MAPK) signaling pathway was responsible for BDNF's effects on spine growth. Slice cultures were transfected with enhanced yellow fluorescent protein (eYFP) by particle-mediated gene transfer, and CA1 pyramidal neurons were imaged by laser-scanning confocal microscopy. We confirmed that BDNF (24 h) increases spine density in apical dendrites of CA1 neurons. The MEK (ERK kinase) inhibitors PD98059 and U0126 completely prevented the increase in spine density induced by BDNF, without having an effect on spine density by themselves. In contrast to its actions on cortical pyramidal neurons, BDNF had minor and rather localized effects on dendritic complexity in hippocampal pyramidal neurons, increasing the total length, but not the branching of apical dendrites within CA1 stratum radiatum, without affecting basal dendrites in stratum oriens. Our results support the hypothesis that the ERK-signaling pathway not only mediates long-term synaptic plasticity and hippocampal-dependent learning, but it is also involved in the structural remodeling of excitatory spine synapses triggered by neurotrophins.
Figures


References
-
- Alessi, D.R., Cuenda, A., Cohen, P., Dudley, D.T., and Saltiel, A.R. 1995. PD098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 270: 27489-27494. - PubMed
-
- Alonso, M., Vianna, M.R.M., Depino, A.M., Mello e Souza, T., Pereira, P., Szapiro, G., Viola, H., Pitossi, F., Izquierdo, I., and Medina, J.H. 2002a. BNDF-triggered events in the rat hippocampus are required for both short-and long-term memory formation. Hippocampus 12: 551-560. - PubMed
-
- Blanquet, P.R. 2000. Identification of two persistently activated neurotrophin-regulated pathways in rat hippocampus. Neuroscience 95: 705-719. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
