Tumour-induced apoptosis in human mesothelial cells: a mechanism of peritoneal invasion by Fas Ligand/Fas interaction
- PMID: 15054468
- PMCID: PMC2409686
- DOI: 10.1038/sj.bjc.6601635
Tumour-induced apoptosis in human mesothelial cells: a mechanism of peritoneal invasion by Fas Ligand/Fas interaction
Abstract
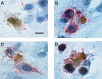
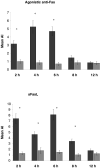
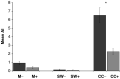
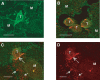
Gastrointestinal carcinomas frequently disseminate within the abdominal cavity to form secondary peritoneal metastases. Invasion of the peritoneal mesothelium is fundamental to this process, yet the underlying invasive mechanisms remain unclear. Preliminary in vitro work suggested that tumour cells can induce mesothelial apoptosis, representing a novel mechanism of peritoneal invasion. We examined the role of tumour cell-induced mesothelial apoptosis and explored the role of the death ligand/receptor system, Fas Ligand/Fas, as mediators of the apoptotic process. Cultured human mesothelial cells were used to establish in vitro co-culture models with the SW480 colonic cancer cell line. Tumour-induced mesothelial apoptosis was confirmed by phase-contrast microscopy and apoptotic detection assays. Human mesothelial cells and SW480 tumour cells constitutively expressed Fas and Fas Ligand mRNA and protein as determined by RT-PCR and confocal fluorescent microscopy. Stimulation of human mesothelial cells with anti-Fas monoclonal antibody or crosslinked soluble Fas Ligand-induced apoptosis, confirming the functional status of the Fas receptor. Pretreatment of SW480 cells with a blocking recombinant anti-Fas Ligand monoclonal antibody significantly reduced mesothelial apoptosis, indicating that tumour-induced mesothelial apoptosis may, in part, be mediated via a Fas-dependent mechanism. This represents a novel mechanism of mesothelial invasion and offers several new targets for therapeutic intervention.
Figures
References
-
- Akedo H, Sinkai K, Mukai M, Mori Y, Tateishi R, Tanaka K, Yamamoto R, Morishita T (1986) Interaction of rat ascites hepatoma cells with cultured mesothelial cell layers. Model for tumour invasion. Cancer Res 46: 2416–2422 - PubMed
-
- Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC (1995) A role for CD95 ligand in preventing graft rejection. Nature 377: 630–632 - PubMed
-
- Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA (1995) Fas ligand-induced apoptosis as a mechanism of immune privilege. Science 270: 1189–1192 - PubMed
-
- Hahne M, Rimoldi D, Schröter M, Romero P, Schreier M, French LE, Schneider P, Bornand T, Fontana A, Lienard D, Cerottini J-C, Tschopp J (1996) Melanoma cell expression of Fas (APO-1/CD95) Ligand: implications for tumor immune escape. Science 274: 1363–1366 - PubMed
-
- Herr I, Posovosky C, Di Marzio L, Cifone MG, Boehler T, Debatin K-M (2000) Autoamplification of apoptosis following ligation of CD95-L, TRAIL and TNF-α. Oncogene 19: 4255–4262 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

