Key role of proline-rich tyrosine kinase 2 in interleukin-8 (CXCL8/IL-8)-mediated human neutrophil chemotaxis
- PMID: 15056377
- PMCID: PMC1782435
- DOI: 10.1111/j.1365-2567.2004.01822.x
Key role of proline-rich tyrosine kinase 2 in interleukin-8 (CXCL8/IL-8)-mediated human neutrophil chemotaxis
Abstract
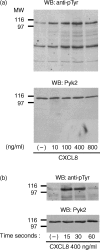
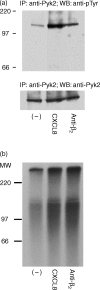
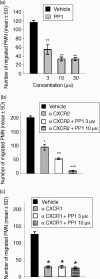
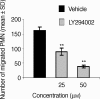
The signalling pathways leading to CXCL8/IL-8-induced human neutrophil migration have not been fully characterized. The present study demonstrates that CXCL8 induces tyrosine phosphorylation as well as enzymatic activity of proline-rich tyrosine kinase 2 (Pyk2), a non-receptor protein tyrosine kinase (PTK), in human neutrophils. Induction of Pyk2 tyrosine phosphorylation by CXCL8 is regulated by Src PTK activation, whereas it is unaffected by phosphatidylinositol 3-kinase activation. Inhibition of Pyk2 activation by PP1, a Src PTK inhibitor, is paralleled by the inhibition of CXCL8-mediated neutrophil chemotaxis. Among CXCL8 receptors, Src protein tyrosine kinase activation selectively regulates CXCR1-mediated polymorphonuclear neutrophil (PMN) chemotaxis. Overexpression of PykM, the kinase-dead mutant of Pyk2, blocks CXCL8-induced chemotaxis of HL-60-derived PMN-like cells, thus pinpointing the key role of Pyk2 in CXCL8-induced chemotaxis.
Figures

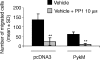
Similar articles
-
Cbl and Akt regulate CXCL8-induced and CXCR1- and CXCR2-mediated chemotaxis.Int Immunol. 2006 Aug;18(8):1315-25. doi: 10.1093/intimm/dxl064. Epub 2006 Jun 23. Int Immunol. 2006. PMID: 16798838
-
Stromal cell-derived factor-1alpha/CXCL12-induced chemotaxis of T cells involves activation of the RasGAP-associated docking protein p62Dok-1.Blood. 2005 Jan 15;105(2):474-80. doi: 10.1182/blood-2004-03-0843. Epub 2004 Sep 2. Blood. 2005. PMID: 15345598
-
PYK2 autophosphorylation, but not kinase activity, is necessary for adhesion-induced association with c-Src, osteoclast spreading, and bone resorption.J Biol Chem. 2003 Mar 28;278(13):11502-12. doi: 10.1074/jbc.M206579200. Epub 2003 Jan 3. J Biol Chem. 2003. PMID: 12514172
-
Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK- cell migration.EMBO J. 1998 Oct 15;17(20):5933-47. doi: 10.1093/emboj/17.20.5933. EMBO J. 1998. PMID: 9774338 Free PMC article.
-
Inhibition of interleukin-8 (CXCL8/IL-8) responses by repertaxin, a new inhibitor of the chemokine receptors CXCR1 and CXCR2.Biochem Pharmacol. 2005 Feb 1;69(3):385-94. doi: 10.1016/j.bcp.2004.10.007. Epub 2004 Dec 9. Biochem Pharmacol. 2005. PMID: 15652230
Cited by
-
Self-sustaining IL-8 loops drive a prothrombotic neutrophil phenotype in severe COVID-19.JCI Insight. 2021 Sep 22;6(18):e150862. doi: 10.1172/jci.insight.150862. JCI Insight. 2021. PMID: 34403366 Free PMC article.
-
Quantitative proteomic analysis of murine white adipose tissue for peritoneal cancer metastasis.Anal Bioanal Chem. 2018 Feb;410(5):1583-1594. doi: 10.1007/s00216-017-0813-9. Epub 2017 Dec 27. Anal Bioanal Chem. 2018. PMID: 29282499 Free PMC article.
-
Bulk RNA sequencing of human pediatric lung cell populations reveals unique transcriptomic signature associated with postnatal pulmonary development.Am J Physiol Lung Cell Mol Physiol. 2024 May 1;326(5):L604-L617. doi: 10.1152/ajplung.00385.2023. Epub 2024 Mar 5. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 38442187 Free PMC article.
-
Inhibition of Pyk2 blocks airway inflammation and hyperresponsiveness in a mouse model of asthma.Am J Respir Cell Mol Biol. 2010 Apr;42(4):491-7. doi: 10.1165/rcmb.2008-0469OC. Epub 2009 Jun 11. Am J Respir Cell Mol Biol. 2010. PMID: 19520918 Free PMC article.
-
Proline-Rich Protein Tyrosine Kinase 2 in Inflammation and Cancer.Cancers (Basel). 2018 May 8;10(5):139. doi: 10.3390/cancers10050139. Cancers (Basel). 2018. PMID: 29738483 Free PMC article. Review.
References
-
- Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines – CXC and CC chemokines. Adv Immunol. 1994;55:97–179. - PubMed
-
- Baggiolini M, Loetscher P. Chemokines in inflammation and immunity. Immunol Today. 2000;21:418–20. - PubMed
-
- Mantovani A. The chemokine system: redundancy for robust outputs. Immunol Today. 1999;20:254–7. - PubMed
-
- Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–7. - PubMed
-
- Ben-Baruch A, Michiel DF, Oppenheim JJ. Signals and receptors involved in recruitment of inflammatory cells. J Biol Chem. 1995;270:11703–6. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
