The interaction of streptococcal inhibitor of complement (SIC) and its proteolytic fragments with the human beta defensins
- PMID: 15056382
- PMCID: PMC1782441
- DOI: 10.1111/j.0019-2805.2004.01837.x
The interaction of streptococcal inhibitor of complement (SIC) and its proteolytic fragments with the human beta defensins
Abstract
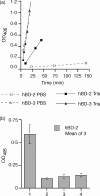
Streptococcal inhibitor of complement (SIC) is a 31 kDa extracellular protein produced by a few highly virulent strains of Streptococcus pyogenes (in particular the M1 strain). It has been shown additionally to inhibit four further components of the mucosal innate response-lysozyme, secretory leucocyte proteinase inhibitor, human alpha-defensin 1 and the cathelicidin LL-37 which are all bactericidal against Group A Streptococci (GAS). We now show that SIC also inhibits variably the antibacterial action of hBD-1, -2 and -3. By enzyme-linked immunosorbent assay (ELISA), SIC binds strongly to hBD-2 and hBD-3, but not at all to hBD-1. Investigation of the antimicrobial action of beta-defensins hBD-1, -2 and -3 against GAS in two different buffer systems shows that both the killing efficiencies of all three defensins, and the binding of SIC to them, occurs more efficiently in 10 mm Tris buffer than in 10 mm phosphate. The lower ionic strength of the Tris buffer may underlie this effect. hBD-1 kills the M1 strain of GAS only in 10 mm Tris, but is able to kill an M6 (SIC negative) strain in 10 mm phosphate. The inhibition of hBD-3 by SIC is clearly of physiological relevance, that of hBD-2 is likely to be so, but the inhibition of hBD-1 occurs only at lower ionic strength than is likely to be encountered in vivo. Elastase digestion of SIC yields three major fragments of MW 3.843 kDa comprising residues 1-33 (fragment A); 10.369 kDa comprising residues 34-126 (fragment B); and MW 16.487 kDa, comprising residues 127-273 (fragment C). By ELISA, only fragment B binds to hBD-2 and hBD-3 and this may indicate the inhibitory portion of the SIC molecule.
Figures






Similar articles
-
Streptococcal DRS (distantly related to SIC) and SIC inhibit antimicrobial peptides, components of mucosal innate immunity: a comparison of their activities.Microbes Infect. 2007 Mar;9(3):300-7. doi: 10.1016/j.micinf.2006.12.006. Epub 2007 Jan 8. Microbes Infect. 2007. PMID: 17303463
-
Streptococcal inhibitor of complement inhibits two additional components of the mucosal innate immune system: secretory leukocyte proteinase inhibitor and lysozyme.Infect Immun. 2002 Sep;70(9):4908-16. doi: 10.1128/IAI.70.9.4908-4916.2002. Infect Immun. 2002. PMID: 12183536 Free PMC article.
-
Attribution of the various inhibitory actions of the streptococcal inhibitor of complement (SIC) to regions within the molecule.J Biol Chem. 2005 May 20;280(20):20120-5. doi: 10.1074/jbc.M414194200. Epub 2005 Mar 15. J Biol Chem. 2005. PMID: 15769742
-
Human defensins.J Mol Med (Berl). 2005 Aug;83(8):587-95. doi: 10.1007/s00109-005-0657-1. Epub 2005 Apr 9. J Mol Med (Berl). 2005. PMID: 15821901 Review.
-
The host and the flora.Dig Dis. 2013;31(3-4):286-92. doi: 10.1159/000354680. Epub 2013 Nov 14. Dig Dis. 2013. PMID: 24246976 Review.
Cited by
-
Horizontal gene transfer and recombination in Streptococcus dysgalactiae subsp. equisimilis.Front Microbiol. 2014 Dec 15;5:676. doi: 10.3389/fmicb.2014.00676. eCollection 2014. Front Microbiol. 2014. PMID: 25566202 Free PMC article.
-
Antimicrobial peptides: agents of border protection for companion animals.Vet Dermatol. 2012 Jun;23(3):177-e36. doi: 10.1111/j.1365-3164.2012.01037.x. Epub 2012 Mar 12. Vet Dermatol. 2012. PMID: 22409270 Free PMC article. Review.
-
Antibiotic development challenges: the various mechanisms of action of antimicrobial peptides and of bacterial resistance.Front Microbiol. 2013 Dec 9;4:353. doi: 10.3389/fmicb.2013.00353. Front Microbiol. 2013. PMID: 24367355 Free PMC article. Review.
-
Extracellular defense of bacteria against antimicrobial peptides.J Bacteriol. 2025 Aug 21;207(8):e0016625. doi: 10.1128/jb.00166-25. Epub 2025 Aug 1. J Bacteriol. 2025. PMID: 40748075 Free PMC article. Review.
-
Categorizing Sequences of Concern by Function To Better Assess Mechanisms of Microbial Pathogenesis.Infect Immun. 2022 May 19;90(5):e0033421. doi: 10.1128/IAI.00334-21. Epub 2021 Nov 15. Infect Immun. 2022. PMID: 34780277 Free PMC article. Review.
References
-
- Åkesson P, Sjöholm AG, Björck L. Protein SIC, a novel extracellular protein of Streptococcus pyogenes interfering with complement function. J Biol Chem. 1996;271:1081–8. - PubMed
-
- Frick I-M, Åkesson P, Rasmussen M, Schmidtchen A, Björck L. SIC—a secreted protein of Streptococcus pyogenes that inactivates antibacterial peptides. J Biol Chem. 2003;278:16561–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
