Neuronal microtubule-associated protein 2D is a dual a-kinase anchoring protein expressed in rat ovarian granulosa cells
- PMID: 15056665
- PMCID: PMC1565147
- DOI: 10.1074/jbc.M402980200
Neuronal microtubule-associated protein 2D is a dual a-kinase anchoring protein expressed in rat ovarian granulosa cells
Abstract
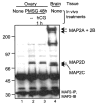
A-kinase anchoring proteins (AKAPs) function to target protein kinase A (PKA) to specific locations within the cell. AKAPs are functionally identified by their ability to bind the type II regulatory subunits (RII) of PKA in an in vitro overlay assay. We previously showed that follicle-stimulating hormone (FSH) induces the expression of an 80-kDa AKAP (AKAP 80) in ovarian granulosa cells as they mature from a preantral to a preovulatory phenotype. In this report, we identify AKAP 80 as microtubule-associated protein 2D (MAP2D), a low molecular weight splice variant of the neuronal MAP2 protein. MAP2D is induced in granulosa cells by dexamethasone and by FSH in a time-dependent manner that mimics that of AKAP 80, and immunoprecipitation of MAP2D depletes extracts of AKAP 80. MAP2D is the only MAP2 protein present in ovaries and is localized to granulosa cells of preovulatory follicles and to luteal cells. MAP2D is concentrated at the Golgi apparatus along with RI and RII and, based on coimmunoprecipitation results, appears to bind both RI and RII in granulosa cells. Reduced expression of MAP2D resulting from treatment of granulosa cells with antisense oligonucleotides to MAP2 inhibited the phosphorylation of cAMP-response element-binding protein. These results suggest that this classic neuronal RII AKAP is a dual RI/RII AKAP that performs unique functions in ovarian granulosa cells that contribute to the preovulatory phenotype.
Figures







Similar articles
-
Dephosphorylation of MAP2D enhances its binding to vimentin in preovulatory ovarian granulosa cells.J Cell Sci. 2016 Aug 1;129(15):2983-96. doi: 10.1242/jcs.190397. Epub 2016 Jun 22. J Cell Sci. 2016. PMID: 27335427 Free PMC article.
-
Luteinizing hormone receptor activation in ovarian granulosa cells promotes protein kinase A-dependent dephosphorylation of microtubule-associated protein 2D.Mol Endocrinol. 2008 Jul;22(7):1695-710. doi: 10.1210/me.2007-0457. Epub 2008 May 8. Mol Endocrinol. 2008. PMID: 18467524 Free PMC article.
-
Identification of cAMP-dependent protein kinase holoenzymes in preantral- and preovulatory-follicle-enriched ovaries, and their association with A-kinase-anchoring proteins.Biochem J. 1999 Dec 1;344 Pt 2(Pt 2):613-23. Biochem J. 1999. PMID: 10567247 Free PMC article.
-
Follicle-stimulating hormone regulation of A-kinase anchoring proteins in granulosa cells.J Biol Chem. 1993 Oct 5;268(28):20729-32. J Biol Chem. 1993. PMID: 8407895
-
Regulation of expression of A-kinase anchoring proteins in rat granulosa cells.Biol Reprod. 1998 Jun;58(6):1496-502. doi: 10.1095/biolreprod58.6.1496. Biol Reprod. 1998. PMID: 9623611
Cited by
-
Proteomic Analysis of Porcine Pre-ovulatory Follicle Differentiation Into Corpus Luteum.Front Endocrinol (Lausanne). 2019 Nov 15;10:774. doi: 10.3389/fendo.2019.00774. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31798533 Free PMC article.
-
Role of the phosphatidylinositol-3-kinase and extracellular regulated kinase pathways in the induction of hypoxia-inducible factor (HIF)-1 activity and the HIF-1 target vascular endothelial growth factor in ovarian granulosa cells in response to follicle-stimulating hormone.Endocrinology. 2009 Feb;150(2):915-28. doi: 10.1210/en.2008-0850. Epub 2008 Oct 9. Endocrinology. 2009. PMID: 18845636 Free PMC article.
-
Luteinizing hormone receptor-stimulated progesterone production by preovulatory granulosa cells requires protein kinase A-dependent activation/dephosphorylation of the actin dynamizing protein cofilin.Mol Endocrinol. 2010 Sep;24(9):1765-81. doi: 10.1210/me.2009-0487. Epub 2010 Jul 7. Mol Endocrinol. 2010. PMID: 20610540 Free PMC article.
-
Dephosphorylation of MAP2D enhances its binding to vimentin in preovulatory ovarian granulosa cells.J Cell Sci. 2016 Aug 1;129(15):2983-96. doi: 10.1242/jcs.190397. Epub 2016 Jun 22. J Cell Sci. 2016. PMID: 27335427 Free PMC article.
-
APPL1, APPL2, Akt2 and FOXO1a interact with FSHR in a potential signaling complex.Mol Cell Endocrinol. 2007 Jan 2;260-262:93-9. doi: 10.1016/j.mce.2006.08.014. Epub 2006 Oct 9. Mol Cell Endocrinol. 2007. PMID: 17030088 Free PMC article.
References
-
- McGee EA, Hsueh AJ. Endocr Rev. 2000;21:200 –214. - PubMed
-
- Richards JS. Physiol Rev. 1980;60:51–89. - PubMed
-
- Robinson-White A, Stratakis CA. Ann N Y Acad Sci. 2002;968:256 –270. - PubMed
-
- Taylor SS. J Biol Chem. 1989;264:8443–8446. - PubMed
-
- Dell’Acqua ML, Scott JD. J Biol Chem. 1997;272:12881–12884. - PubMed

