Potassium model for slow (2-3 Hz) in vivo neocortical paroxysmal oscillations
- PMID: 15056684
- PMCID: PMC2925854
- DOI: 10.1152/jn.00529.2003
Potassium model for slow (2-3 Hz) in vivo neocortical paroxysmal oscillations
Abstract
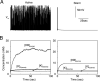
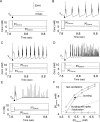
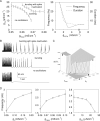
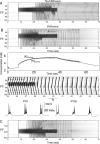
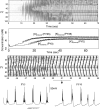
In slow neocortical paroxysmal oscillations, the de- and hyperpolarizing envelopes in neocortical neurons are large compared with slow sleep oscillations. Increased local synchrony of membrane potential oscillations during seizure is reflected in larger electroencephalographic oscillations and the appearance of spike- or polyspike-wave complex recruitment at 2- to 3-Hz frequencies. The oscillatory mechanisms underlying this paroxysmal activity were investigated in computational models of cortical networks. The extracellular K(+) concentration ([K(+)](o)) was continuously computed based on neuronal K(+) currents and K(+) pumps as well as glial buffering. An increase of [K(+)](o) triggered a transition from normal awake-like oscillations to 2- to 3-Hz seizure-like activity. In this mode, the cells fired periodic bursts and nearby neurons oscillated highly synchronously; in some cells depolarization led to spike inactivation lasting 50-100 ms. A [K(+)](o) increase, sufficient to produce oscillations could result from excessive firing (e.g., induced by external stimulation) or inability of K(+) regulatory system (e.g., when glial buffering was blocked). A combination of currents including high-threshold Ca(2+), persistent Na(+) and hyperpolarization-activated depolarizing (I(h)) currents was sufficient to maintain 2- to 3-Hz activity. In a network model that included lateral K(+) diffusion between cells, increase of [K(+)](o) in a small region was generally sufficient to maintain paroxysmal oscillations in the whole network. Slow changes of [K(+)](o) modulated the frequency of bursting and, in some case, led to fast oscillations in the 10- to 15-Hz frequency range, similar to the fast runs observed during seizures in vivo. These results suggest that modifications of the intrinsic currents mediated by increase of [K(+)](o) can explain the range of neocortical paroxysmal oscillations in vivo.
Figures



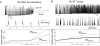


References
-
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–224. - PubMed
-
- Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Cellular and network models for intrathalamic augmenting responses during 10-Hz stimulation. J Neurophysiol. 1998;79:2730–2748. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

