Parallel FoxP1 and FoxP2 expression in songbird and human brain predicts functional interaction
- PMID: 15056695
- PMCID: PMC6730014
- DOI: 10.1523/JNEUROSCI.5589-03.2004
Parallel FoxP1 and FoxP2 expression in songbird and human brain predicts functional interaction
Abstract
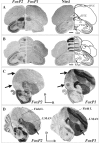
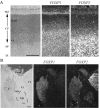
Humans and songbirds are two of the rare animal groups that modify their innate vocalizations. The identification of FOXP2 as the monogenetic locus of a human speech disorder exhibited by members of the family referred to as KE enables the first examination of whether molecular mechanisms for vocal learning are shared between humans and songbirds. Here, in situ hybridization analyses for FoxP1 and FoxP2 in a songbird reveal a corticostriatal expression pattern congruent with the abnormalities in brain structures of affected KE family members. The overlap in FoxP1 and FoxP2 expression observed in the songbird suggests that combinatorial regulation by these molecules during neural development and within vocal control structures may occur. In support of this idea, we find that FOXP1 and FOXP2 expression patterns in human fetal brain are strikingly similar to those in the songbird, including localization to subcortical structures that function in sensorimotor integration and the control of skilled, coordinated movement. The specific colocalization of FoxP1 and FoxP2 found in several structures in the bird and human brain predicts that mutations in FOXP1 could also be related to speech disorders.
Figures






Similar articles
-
Expression analysis of the speech-related genes FoxP1 and FoxP2 and their relation to singing behavior in two songbird species.J Exp Biol. 2013 Oct 1;216(Pt 19):3682-92. doi: 10.1242/jeb.085886. J Exp Biol. 2013. PMID: 24006346 Free PMC article.
-
Characterization of Foxp2 and Foxp1 mRNA and protein in the developing and mature brain.J Comp Neurol. 2003 May 26;460(2):266-79. doi: 10.1002/cne.10654. J Comp Neurol. 2003. PMID: 12687690
-
From songbird to humans: The multifaceted roles of FOXP2 in speech and motor learning.Neurosci Biobehav Rev. 2024 Dec;167:105936. doi: 10.1016/j.neubiorev.2024.105936. Epub 2024 Nov 6. Neurosci Biobehav Rev. 2024. PMID: 39510218 Review.
-
Differential FoxP2 and FoxP1 expression in a vocal learning nucleus of the developing budgerigar.Dev Neurobiol. 2015 Jul;75(7):778-90. doi: 10.1002/dneu.22247. Epub 2014 Nov 26. Dev Neurobiol. 2015. PMID: 25407828 Free PMC article.
-
Genetic components of vocal learning.Ann N Y Acad Sci. 2004 Jun;1016:325-47. doi: 10.1196/annals.1298.032. Ann N Y Acad Sci. 2004. PMID: 15313783 Review.
Cited by
-
Genetic insights into the functional elements of language.Hum Genet. 2013 Sep;132(9):959-86. doi: 10.1007/s00439-013-1317-0. Epub 2013 Jun 8. Hum Genet. 2013. PMID: 23749164 Review.
-
The distinct and overlapping phenotypic spectra of FOXP1 and FOXP2 in cognitive disorders.Hum Genet. 2012 Nov;131(11):1687-98. doi: 10.1007/s00439-012-1193-z. Epub 2012 Jun 27. Hum Genet. 2012. PMID: 22736078 Free PMC article. Review.
-
Neural FoxP2 and FoxP1 expression in the budgerigar, an avian species with adult vocal learning.Behav Brain Res. 2015 Apr 15;283:22-9. doi: 10.1016/j.bbr.2015.01.017. Epub 2015 Jan 16. Behav Brain Res. 2015. PMID: 25601574 Free PMC article.
-
An avian cortical circuit for chunking tutor song syllables into simple vocal-motor units.Nat Commun. 2020 Oct 6;11(1):5029. doi: 10.1038/s41467-020-18732-x. Nat Commun. 2020. PMID: 33024101 Free PMC article.
-
Distribution of language-related Cntnap2 protein in neural circuits critical for vocal learning.J Comp Neurol. 2014 Jan 1;522(1):169-85. doi: 10.1002/cne.23394. J Comp Neurol. 2014. PMID: 23818387 Free PMC article.
References
-
- Akutagawa E, Konishi M (2001) A monoclonal antibody specific to a song system nuclear antigen in estrildine finches. Neuron 31: 545–556. - PubMed
-
- Alcock KJ, Passingham RE, Watkins KE, Vargha-Khadem F (2000a) Oral dyspraxia in inherited speech and language impairment and acquired dysphasia. Brain Lang 75: 17–33. - PubMed
-
- Alcock KJ, Passingham RE, Watkins K, Vargha-Khadem F (2000b) Pitch and timing abilities in inherited speech and language impairment. Brain Lang 75: 34–46. - PubMed
-
- Bottjer SW, Johnson F (1997) Circuits, hormones, and learning: vocal behavior in songbirds. J Neurobiol 33: 602–618. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
