Connexin32-containing gap junctions in Schwann cells at the internodal zone of partial myelin compaction and in Schmidt-Lanterman incisures
- PMID: 15056698
- PMCID: PMC1803337
- DOI: 10.1523/JNEUROSCI.5146-03.2004
Connexin32-containing gap junctions in Schwann cells at the internodal zone of partial myelin compaction and in Schmidt-Lanterman incisures
Abstract
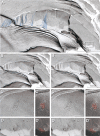
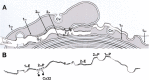
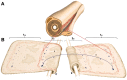
In vertebrate peripheral nerves, the insulating myelin sheath is formed by Schwann cells, which generate flattened membrane processes that spiral around axons and form compact myelin by extrusion of cytoplasm and adhesion of apposed intracellular and extracellular membrane surfaces. Cytoplasm remains within the innermost and outermost tongues, in the paranodal loops bordering nodes of Ranvier and in Schmidt-Lanterman incisures. By immunocytochemistry, connexin32 (Cx32) protein has been demonstrated at paranodal loops and Schmidt-Lanterman incisures, and it is widely assumed that gap junctions are present in these locations, thereby providing a direct radial route for transport of ions and metabolites between cytoplasmic myelin layers. This study used freeze-fracture replica immunogold labeling to detect Cx32 in ultrastructurally defined gap junctions in Schmidt-Lanterman incisures, as well as in a novel location, between the outer two layers of internodal myelin, approximately every micrometer along the entire length of myelin, at the zone between compact myelin and noncompact myelin. Thus, these gap junctions link the partially compacted second layer of myelin to the noncompact outer tongue. Although these gap junctions are unusually small (average, 11 connexon channels), their relative abundance and regular distribution along the zone that is structurally intermediate between compact and noncompact myelin demonstrates the existence of multiple sites for unidirectional or bidirectional transport of water, ions, and small molecules between these two distinct cytoplasmic compartments, possibly to regulate or facilitate myelin compaction or to maintain the transition zone between noncompact and compact myelin.
Figures




References
-
- Bergoffen J, Scherer SS, Wang S, Oronzi-Scott M, Bone L, Paul DL, Chen K, Lensch MW, Chance P, Fischbeck K (1993) Connexin mutations in X-linked Charcot-Marie-Tooth disease. Science 262: 2039–2042. - PubMed
-
- Branton D, Bullivant S, Gilula NB, Karnovsky MJ, Moor H, Muhlethaler K, Northcote DH, Packer L, Satir B, Satir P, Speth V, Staehlin LA, Steere RL, Weinstein RS (1975) Freeze-etching nomenclature. Science 190: 54–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
