Common sensory inputs and differential excitability of segmentally homologous reticulospinal neurons in the hindbrain
- PMID: 15056699
- PMCID: PMC6730040
- DOI: 10.1523/JNEUROSCI.4419-03.2004
Common sensory inputs and differential excitability of segmentally homologous reticulospinal neurons in the hindbrain
Abstract
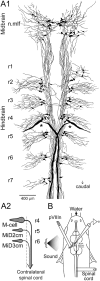
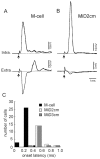
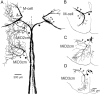
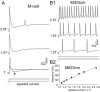
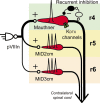
In the hindbrain of zebrafish and goldfish, reticulospinal (RS) neurons are arranged in seven segments, with segmental homologs in adjacent segments. The Mauthner cell (M-cell) in the fourth segment (r4) is known to trigger fast escape behavior. Its serial homologs, MiD2cm in r5 and MiD3cm in r6, are predicted to contribute to this behavior, which can be evoked by head-tap stimuli. However, little is known about their input-output properties. Therefore, we studied afferent projections from the auditory posterior eighth nerve (pVIIIn) and firing properties of MiD2cm and MiD3cm for comparison with the M-cell in adult goldfish. Labeling of RS neurons and the pVIIIn afferents with fluorescent tracers showed that the pVIIIn projected to r4-r6. Tone burst and electrical stimulation of the pVIIIn evoked EPSPs in the M-cell, MiD2cm, and MiD3cm. Stepwise depolarization typically elicited a single spike at the onset in the M-cell but repetitive spiking in MiD2cm and MiD3cm. This atypical property of the M-cell was mediated by dendrotoxin-I (DTX-I)-sensitive voltage-gated potassium channels together with recurrent inhibition, because combined application of DTX-I, strychnine, and bicuculline led to continuous repetitive firing in M-cells. The M-cell but not MiD2cm or MiD3cm expressed Kv1.2, a DTX-I-sensitive potassium channel subunit. Thus, the M-cell and its segmental homologs may sense common auditory information but send different outputs to the spinal circuits to control adaptive escape behavior.
Figures






Similar articles
-
Coexpression of auxiliary Kvβ2 subunits with Kv1.1 channels is required for developmental acquisition of unique firing properties of zebrafish Mauthner cells.J Neurophysiol. 2014 Mar;111(6):1153-64. doi: 10.1152/jn.00596.2013. Epub 2013 Dec 11. J Neurophysiol. 2014. PMID: 24335214
-
[Functional organization of escape circuits built in teleost hindbrain segments].Nihon Shinkei Seishin Yakurigaku Zasshi. 2008 Jun;28(3):127-30. Nihon Shinkei Seishin Yakurigaku Zasshi. 2008. PMID: 18646598 Review. Japanese.
-
Functional motifs composed of morphologically homologous neurons repeated in the hindbrain segments.J Neurosci. 2014 Feb 26;34(9):3291-302. doi: 10.1523/JNEUROSCI.4610-13.2014. J Neurosci. 2014. PMID: 24573288 Free PMC article.
-
[Functional organization of segmentally homologous reticulospinal neurons in hindbrain].Tanpakushitsu Kakusan Koso. 2004 Feb;49(3 Suppl):486-92. Tanpakushitsu Kakusan Koso. 2004. PMID: 14976777 Review. Japanese. No abstract available.
-
Coordinated Expression of Two Types of Low-Threshold K+ Channels Establishes Unique Single Spiking of Mauthner Cells among Segmentally Homologous Neurons in the Zebrafish Hindbrain.eNeuro. 2017 Oct 23;4(5):ENEURO.0249-17.2017. doi: 10.1523/ENEURO.0249-17.2017. eCollection 2017 Sep-Oct. eNeuro. 2017. PMID: 29085904 Free PMC article.
Cited by
-
Graph theoretical model of a sensorimotor connectome in zebrafish.PLoS One. 2012;7(5):e37292. doi: 10.1371/journal.pone.0037292. Epub 2012 May 18. PLoS One. 2012. PMID: 22624008 Free PMC article.
-
Neural representation of object approach in a decision-making motor circuit.J Neurosci. 2006 Mar 29;26(13):3454-64. doi: 10.1523/JNEUROSCI.5259-05.2006. J Neurosci. 2006. PMID: 16571752 Free PMC article.
-
Mapping a sensory-motor network onto a structural and functional ground plan in the hindbrain.Proc Natl Acad Sci U S A. 2011 Jan 18;108(3):1170-5. doi: 10.1073/pnas.1012189108. Epub 2011 Jan 3. Proc Natl Acad Sci U S A. 2011. PMID: 21199937 Free PMC article.
-
The Formin Fmn2b Is Required for the Development of an Excitatory Interneuron Module in the Zebrafish Acoustic Startle Circuit.eNeuro. 2021 Jul 9;8(4):ENEURO.0329-20.2021. doi: 10.1523/ENEURO.0329-20.2021. Print 2021 Jul-Aug. eNeuro. 2021. PMID: 34193512 Free PMC article.
-
4-dimensional functional profiling in the convulsant-treated larval zebrafish brain.Sci Rep. 2017 Jul 26;7(1):6581. doi: 10.1038/s41598-017-06646-6. Sci Rep. 2017. PMID: 28747660 Free PMC article.
References
-
- Bal R, Oertel D (2001) Potassium currents in octopus cells of the mammalian cochlear nucleus. J Neurophysiol 86: 2299–2311. - PubMed
-
- Bosch TJ, Maslam S, Roberts BL (2001) Fos-like immunohistochemical identification of neurons active during the startle response of the rainbow trout. J Comp Neurol 439: 306–314. - PubMed
-
- Canfield JG, Eaton RC (1990) Swimbladder acoustic pressure transduction initiates Mauthner-mediated escape. Nature 347: 760–761.
-
- Casagrand JL, Guzik AL, Eaton RC (1999) Mauthner and reticulospinal responses to the onset of acoustic pressure and acceleration stimuli. J Neurophysiol 82: 1422–1437. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
