Representation of angles embedded within contour stimuli in area V2 of macaque monkeys
- PMID: 15056711
- PMCID: PMC6730022
- DOI: 10.1523/JNEUROSCI.4364-03.2004
Representation of angles embedded within contour stimuli in area V2 of macaque monkeys
Abstract
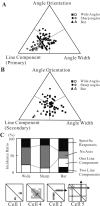
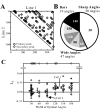
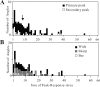
Angles and junctions embedded within contours are important features to represent the shape of objects. To study the neuronal basis to extract these features, we conducted extracellular recordings while two macaque monkeys performed a fixation task. Angle stimuli were the combination of two straight half-lines larger than the size of the classical receptive fields (CRFs). Each line was drawn from the center to outside the CRFs in 1 of 12 directions, so that the stimuli passed through the CRFs and formed angles at the center of the CRFs. Of 114 neurons recorded from the superficial layer of area V2, 91 neurons showed selective responses to these angle stimuli. Of these, 41 neurons (36.0%) showed selective responses to wide angles between 60 degrees and 150 degrees that were distinct from responses to straight lines or sharp angles (30 degrees ). Responses were highly selective to a particular angle in approximately one-fourth of neurons. When we tested the selectivity of the same neurons to individual half-lines, the preferred direction was more or less consistent with one or two components of the optimal angle stimuli. These results suggest that the selectivity of the neurons depends on both the combination of two components and the responses to individual components. Angle-selective V2 neurons are unlikely to be specific angle detectors, because the magnitude of their responses to the optimal angle was indistinguishable from that to the optimal half-lines. We suggest that the extraction of information of angles embedded within contour stimuli may start in area V2.
Figures







References
-
- Allman J, Miezin F, McGuinnes E (1985) Stimulus specific responses from beyond the classical receptive field: neurophysiological mechanisms for local-global comparisons in visual neurons. Annu Rev Neurosci 8: 407–430. - PubMed
-
- Anzai A, Van Essen DC (2001) Receptive field substructure of monkey V2 neurons in the orientation domain. Soc Neurosci Abstr 27: 286.5.
-
- Anzai A, Van Essen DC (2002) Receptive field structure of orientation selective cells in monkey V2. Soc Neurosci Abstr 28: 720.12.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
