Differential distribution and function of hyperpolarization-activated channels in sensory neurons and mechanosensitive fibers
- PMID: 15056713
- PMCID: PMC6730026
- DOI: 10.1523/JNEUROSCI.5156-03.2004
Differential distribution and function of hyperpolarization-activated channels in sensory neurons and mechanosensitive fibers
Abstract
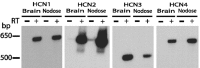
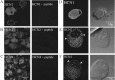
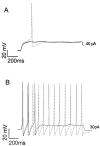
Sensory neurons express hyperpolarization-activated currents (I(H)) that differ in magnitude and kinetics within the populations. We investigated the structural basis for these differences and explored the functional role of the I(H) channels in sensory neurons isolated from rat nodose ganglia. Immunohistochemical studies demonstrated a differential distribution of hyperpolarization-activated cyclic nucleotide-gated (HCN) protein (HCN1, HCN2, HCN4) in sensory neurons and peripheral terminals. HCN2 and HCN4 immunoreactivity was present in all nodose neurons. In contrast, only 20% of the total population expressed HCN1 immunoreactivity. HCN1 did not colocalize with IB4 (a marker for C-type neurons), and only 15% of HCN1-positive neurons colocalized with immunoreactivity for the vanilloid receptor VR1, another protein associated primarily with C-type neurons. Therefore, most HCN1-containing neurons were A-type neurons. In further support, HCN1 was present in the mechanosensitive terminals of myelinated but not unmyelinated sensory fibers, whereas HCN2 and HCN4 were present in receptor terminals of both myelinated and unmyelinated fibers. In voltage-clamp studies, cell permeant cAMP analogs shifted the activation curve for I(H) to depolarized potentials in C-type neurons but not A-type neurons. In current-clamp recording, CsCl, which inhibits only I(H) in nodose neurons, hyperpolarized the resting membrane potential from -63 +/- 1 to -73 +/- 2 mV and nearly doubled the input resistance from 1.3 to 2.2 GOmega. In addition, action potentials were initiated at lower depolarizing current injections in the presence of CsCl. At the sensory receptor terminal, CsCl decreased the threshold pressure for initiation of mechanoreceptor discharge. Therefore, elimination of the I(H) increases excitability of both the soma and the peripheral sensory terminals.
Figures









Similar articles
-
Functional impact of the hyperpolarization-activated current on the excitability of myelinated A-type vagal afferent neurons in the rat.Clin Exp Pharmacol Physiol. 2010 Aug;37(8):852-61. doi: 10.1111/j.1440-1681.2010.05396.x. Epub 2010 Apr 26. Clin Exp Pharmacol Physiol. 2010. PMID: 20456426 Free PMC article.
-
Involvement of hyperpolarization-activated, cyclic nucleotide-gated cation channels in dorsal root ganglion in neuropathic pain.Sheng Li Xue Bao. 2008 Oct 25;60(5):579-80. Sheng Li Xue Bao. 2008. PMID: 18958363
-
Molecular and functional analysis of hyperpolarisation-activated nucleotide-gated (HCN) channels in the enteric nervous system.Neuroscience. 2004;129(3):603-14. doi: 10.1016/j.neuroscience.2004.08.027. Neuroscience. 2004. PMID: 15541882
-
Differential distribution of voltage-gated channels in myelinated and unmyelinated baroreceptor afferents.Auton Neurosci. 2012 Dec 24;172(1-2):4-12. doi: 10.1016/j.autneu.2012.10.014. Epub 2012 Nov 10. Auton Neurosci. 2012. PMID: 23146622 Review.
-
HCN channels as targets for drug discovery.Comb Chem High Throughput Screen. 2009 Jan;12(1):64-72. doi: 10.2174/138620709787048028. Comb Chem High Throughput Screen. 2009. PMID: 19149492 Review.
Cited by
-
Vascular mechanotransduction.Physiol Rev. 2023 Apr 1;103(2):1247-1421. doi: 10.1152/physrev.00053.2021. Epub 2023 Jan 5. Physiol Rev. 2023. PMID: 36603156 Free PMC article. Review.
-
Functional impact of the hyperpolarization-activated current on the excitability of myelinated A-type vagal afferent neurons in the rat.Clin Exp Pharmacol Physiol. 2010 Aug;37(8):852-61. doi: 10.1111/j.1440-1681.2010.05396.x. Epub 2010 Apr 26. Clin Exp Pharmacol Physiol. 2010. PMID: 20456426 Free PMC article.
-
The unsilent majority-TRPV1 drives "spontaneous" transmission of unmyelinated primary afferents within cardiorespiratory NTS.Am J Physiol Regul Integr Comp Physiol. 2012 Dec 15;303(12):R1207-16. doi: 10.1152/ajpregu.00398.2012. Epub 2012 Oct 17. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 23076872 Free PMC article. Review.
-
Independent transmission of convergent visceral primary afferents in the solitary tract nucleus.J Neurophysiol. 2013 Jan;109(2):507-17. doi: 10.1152/jn.00726.2012. Epub 2012 Oct 31. J Neurophysiol. 2013. PMID: 23114206 Free PMC article.
-
Cannabinoid type 1 and type 2 receptor antagonists prevent attenuation of serotonin-induced reflex apneas by dronabinol in Sprague-Dawley rats.PLoS One. 2014 Oct 28;9(10):e111412. doi: 10.1371/journal.pone.0111412. eCollection 2014. PLoS One. 2014. PMID: 25350456 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
