Beta1-integrins are critical for cerebellar granule cell precursor proliferation
- PMID: 15056720
- PMCID: PMC2693074
- DOI: 10.1523/JNEUROSCI.5241-03.2004
Beta1-integrins are critical for cerebellar granule cell precursor proliferation
Abstract
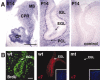
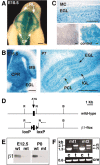
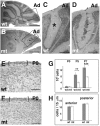
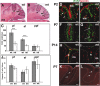
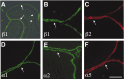
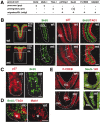
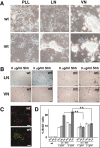
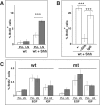
We have previously shown that mice with a CNS restricted knock-out of the integrin beta1 subunit gene (Itgb1-CNSko mice) have defects in the formation of lamina and folia in the cerebral and cerebellar cortices that are caused by disruption of the cortical marginal zones. Cortical structures in postnatal and adult Itgb1-CNSko animals are also reduced in size, but the mechanism that causes the size defect has remained unclear. We now demonstrate that proliferation of granule cell precursors (GCPs) is severely affected in the developing cerebellum of Itgb1-CNSko mice. In the absence of beta1 expression, GCPs lose contact with laminin in the meningeal basement membrane, cease proliferating, and differentiate prematurely. In vitro studies provide evidence that beta1 integrins act at least in part cell autonomously in GCPs to regulate their proliferation. Previous studies have shown that sonic hedgehog (Shh)-induced GCP proliferation is potentiated by the integrin ligand laminin. We show that Shh directly binds to laminin and that laminin-Shh induced cell proliferation is dependent on beta1 integrin expression in GCPs. Taken together, these data are consistent with a model in which beta1 integrin expression in GCPs is required to recruit a laminin-Shh complex to the surface of GCPs and to subsequently modulate the activity of signaling pathways that regulate proliferation.
Figures
References
-
- Altman J, Bayer SA (1997) Development of the cerebellar system in relation to its evolution, structure, and function. New York: CRC.
-
- Baeg GH, Perrimon N (2000) Functional binding of secreted molecules to heparan sulfate proteoglycans in Drosophila Curr Opin Cell Biol 12: 575–580. - PubMed
-
- Barakat I, Wittendorp-Rechenmann W, Rechenmann RV, Sensenbrenner M (1981) Influence of meningeal cells on the proliferation of neuroblasts in culture. Dev Neurosci 4: 363–372. - PubMed
-
- Bellaiche Y, The I, Perrimon N (1998) Tout-velu is a Drosophila homologue of the putative tumour suppressor EXT-1 and is needed for Hh diffusion. Nature 394: 85–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
