Contribution of calcium ions to P2X channel responses
- PMID: 15056721
- PMCID: PMC6730036
- DOI: 10.1523/JNEUROSCI.5429-03.2004
Contribution of calcium ions to P2X channel responses
Abstract
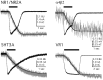
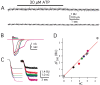
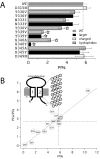
Ca2+ entry through transmitter-gated cation channels, including ATP-gated P2X channels, contributes to an array of physiological processes in excitable and non-excitable cells, but the absolute amount of Ca2+ flowing through P2X channels is unknown. Here we address the issue of precisely how much Ca2+ flows through P2X channels and report the finding that the ATP-gated P2X channel family has remarkably high Ca2+ flux compared with other channels gated by the transmitters ACh, serotonin, protons, and glutamate. Several homomeric and heteromeric P2X channels display fractional Ca2+ currents equivalent to NMDA channels, which hitherto have been thought of as the largest source of transmitter-activated Ca2+ flux. We further suggest that NMDA and P2X channels may use different mechanisms to promote Ca2+ flux across membranes. We find that mutating three critical polar amino acids decreases the Ca2+ flux of P2X2 receptors, suggesting that these residues cluster to form a novel type of Ca2+ selectivity region within the pore. Overall, our data identify P2X channels as a large source of transmitter-activated Ca2+ influx at resting membrane potentials and support the hypothesis that polar amino acids contribute to Ca2+ selection in an ATP-gated ion channel.
Figures

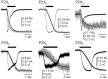


References
-
- Alonzo MT, Chamero P, Villalobos C, Garcia-Sancho J (2003) Fura-2 antagonises calcium-induced calcium release. Cell Calcium 33: 27–35. - PubMed
-
- Benham CD, Tsien RW (1987) A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature 328: 275–278. - PubMed
-
- Berridge MJ, Bootman MD, Roderick HL (2003) Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4: 517–529. - PubMed
-
- Burnashev N (1998) Calcium permeability of ligand-gated channels. Cell Calcium 24: 325–332. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
