Arthropod 5-HT2 receptors: a neurohormonal receptor in decapod crustaceans that displays agonist independent activity resulting from an evolutionary alteration to the DRY motif
- PMID: 15056722
- PMCID: PMC6730010
- DOI: 10.1523/JNEUROSCI.0062-04.2004
Arthropod 5-HT2 receptors: a neurohormonal receptor in decapod crustaceans that displays agonist independent activity resulting from an evolutionary alteration to the DRY motif
Erratum in
- J Neurosci. 2004 May 5;24(18):4489
Abstract
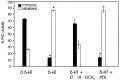
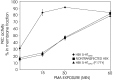
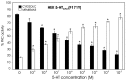
The stomatogastric nervous system (STNS) is a premiere model for studying modulation of motor pattern generation. Whereas the cellular and network responses to monoamines have been particularly well characterized electrophysiologically, the transduction mechanisms that link the different monoaminergic signals to specific intracellular responses are presently unknown in this system. To begin to elucidate monoaminergic signal transduction in pyloric neurons, we used a bioinformatics approach to predict the existence of 18 monoamine receptors in arthropods, 9 of which have been previously cloned in Drosophila and other insects. We then went on to use the two existing insect databases to clone and characterize the 10th putative arthropod receptor from the spiny lobster, Panulirus interruptus. This receptor is most homologous to the 5-HT2 subtype and shows a dose-dependent response to 5-HT but not to any of the other monoamines present in the STNS. Through a series of pharmacological experiments, we demonstrate that this newly described receptor, 5-HT2betaPan, couples with the traditional G(q) pathway when expressed in HEK293 cells, but not to G(s) or G(i/o). Moreover, it is constitutively active, because the highly conserved DRY motif in transmembrane region 3 has evolved into DRF. Site-directed mutagenesis that reverts the motif back to DRY abolishes this agonist-independent activity. We further demonstrate that this receptor most likely participates in the modulation of stomatogastric motor output, because it is found in neurites in the synaptic neuropil of the stomatogastric ganglion as well as in the axon terminals at identified pyloric neuromuscular junctions.
Figures







Similar articles
-
Conservation of structure, signaling and pharmacology between two serotonin receptor subtypes from decapod crustaceans, Panulirus interruptus and Procambarus clarkii.J Exp Biol. 2008 Jan;211(Pt 1):92-105. doi: 10.1242/jeb.012450. J Exp Biol. 2008. PMID: 18083737 Free PMC article.
-
Molecular cloning and characterization of crustacean type-one dopamine receptors: D1alphaPan and D1betaPan.Comp Biochem Physiol B Biochem Mol Biol. 2006 Mar;143(3):294-301. doi: 10.1016/j.cbpb.2005.11.017. Epub 2006 Jan 19. Comp Biochem Physiol B Biochem Mol Biol. 2006. PMID: 16426885 Free PMC article.
-
Mutagenesis analysis of the serotonin 5-HT2C receptor and a Caenorhabditis elegans 5-HT2 homologue: conserved residues of helix 4 and helix 7 contribute to agonist-dependent activation of 5-HT2 receptors.J Neurochem. 2005 Jan;92(2):375-87. doi: 10.1111/j.1471-4159.2004.02867.x. J Neurochem. 2005. PMID: 15663485
-
Serotonin transduction cascades mediate variable changes in pyloric network cycle frequency in response to the same modulatory challenge.J Neurophysiol. 2008 Jun;99(6):2844-63. doi: 10.1152/jn.00986.2007. Epub 2008 Apr 9. J Neurophysiol. 2008. PMID: 18400960
-
[Signal transduction of cloned opioid receptors].Nihon Yakurigaku Zasshi. 1994 Sep;104(3):229-39. doi: 10.1254/fpj.104.229. Nihon Yakurigaku Zasshi. 1994. PMID: 7959415 Review. Japanese.
Cited by
-
Adult neurogenesis in the decapod crustacean brain: a hematopoietic connection?Eur J Neurosci. 2011 Sep;34(6):870-83. doi: 10.1111/j.1460-9568.2011.07802.x. Eur J Neurosci. 2011. PMID: 21929622 Free PMC article. Review.
-
Serotonin in Animal Cognition and Behavior.Int J Mol Sci. 2020 Feb 28;21(5):1649. doi: 10.3390/ijms21051649. Int J Mol Sci. 2020. PMID: 32121267 Free PMC article. Review.
-
Cell specific dopamine modulation of the transient potassium current in the pyloric network by the canonical D1 receptor signal transduction cascade.J Neurophysiol. 2010 Aug;104(2):873-84. doi: 10.1152/jn.00195.2010. Epub 2010 Jun 2. J Neurophysiol. 2010. PMID: 20519576 Free PMC article.
-
Conservation of structure, signaling and pharmacology between two serotonin receptor subtypes from decapod crustaceans, Panulirus interruptus and Procambarus clarkii.J Exp Biol. 2008 Jan;211(Pt 1):92-105. doi: 10.1242/jeb.012450. J Exp Biol. 2008. PMID: 18083737 Free PMC article.
-
Cloning and immunoreactivity of the 5-HT 1Mac and 5-HT 2Mac receptors in the central nervous system of the freshwater prawn Macrobrachium rosenbergii.J Comp Neurol. 2009 Apr 1;513(4):399-416. doi: 10.1002/cne.21979. J Comp Neurol. 2009. PMID: 19184976 Free PMC article.
References
-
- Agretti P, De Marco G, Collecchi P, Chiovato L, Vitti P, Pinchera A, Tonacchera M (2003) Proper targeting and activity of a nonfunctioning thyroid-stimulating hormone receptor (TSHr) combining an inactivating and activating TSHr mutation in one receptor. Eur J Biochem 270: 3839–3847. - PubMed
-
- Almaula N, Ebersole BJ, Ballesteros JA, Weinstein H, Sealfon SC (1996a) Contribution of a helix 5 locus to selectivity of hallucinogenic and non-hallucinogenic ligands for the human 5-hydroxytryptamine2A and 5-hydroxytryptamine2C receptors: direct and indirect effects on ligand affinity mediated by the same locus. Mol Pharmacol 50: 34–42. - PubMed
-
- Almaula N, Ebersole BJ, Zhang D, Weinstein H, Sealfon SC (1996b) Mapping the binding site pocket of the serotonin 5-hydroxytryptamine2A receptor. Ser3.36(159) provides a second interaction site for the protonated amine of serotonin but not of lysergic acid diethylamide or bufotenin. J Biol Chem 271: 14672–14675. - PubMed
-
- Angers S, Salahpour A, Bouvier M (2002) Dimerization: an emerging concept for G protein-coupled receptor ontogeny and function. Annu Rev Pharmacol Toxicol 42: 409–435. - PubMed
-
- Ango F, Prezeau L, Muller T, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, Fagni L (2001) Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature 411: 962–965. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
