Myosin V processivity: multiple kinetic pathways for head-to-head coordination
- PMID: 15056760
- PMCID: PMC397419
- DOI: 10.1073/pnas.0307247101
Myosin V processivity: multiple kinetic pathways for head-to-head coordination
Abstract
Myosin V, a double-headed molecular motor, transports organelles within cells by walking processively along actin, a process that requires coordination between the heads. To understand the mechanism underlying this coordination, processive runs of single myosin V molecules were perturbed by varying nucleotide content. Contrary to current views, our results show that the two heads of a myosin V molecule communicate, not through any one mechanism but through an elaborate system of cooperative mechanisms involving multiple kinetic pathways. These mechanisms introduce redundancy and safeguards that ensure robust processivity under differing physiologic demands.
Figures
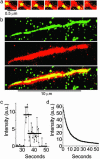
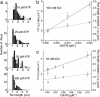
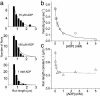
References
-
- Mehta, A. D., Rock, R. S., Rief, M., Spudich, J. A., Mooseker, M. S. & Cheney, R. E. (1999) Nature 400, 590–593. - PubMed
-
- Sakamoto, T., Amitani, I., Yokota, E. & Ando, T. (2000) Biochem. Biophys. Res. Commun. 272, 586–590. - PubMed
-
- Pastural, E., Barrat, F. J., Dufourcq-Lagelouse, R., Certain, S., Sanal, O., Jabado, N., Seger, R., Griscelli, C., Fischer, A. & de Saint, B. G. (1997) Nat. Genet. 16, 289–292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

