Transcutaneous immunization induces mucosal CTLs and protective immunity by migration of primed skin dendritic cells
- PMID: 15057306
- PMCID: PMC379323
- DOI: 10.1172/JCI20261
Transcutaneous immunization induces mucosal CTLs and protective immunity by migration of primed skin dendritic cells
Abstract
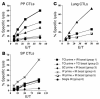
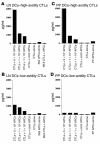
Transcutaneous immunization (TCI), the application of vaccines on the skin, induces robust systemic and mucosal antibodies in animal models and in humans. The means by which mucosal immune responses to vaccine antigens are elicited by TCI has not been well characterized. We examined the effect of TCI with an HIV peptide vaccine on the induction of mucosal and systemic CTL responses and protective immunity against mucosal challenge with live virus in mice. Robust HIV-specific CTL responses in the spleen and in the gut mucosa were detected after TCI. The responses were dependent upon the addition of an adjuvant and resulted in protection against mucosal challenge with recombinant vaccinia virus encoding HIV gp160. Although it is clear that adjuvant-activated DCs migrated mainly to draining lymph nodes, coculture with specific T cells and flow cytometry studies with DCs isolated from Peyer's patches after TCI suggested that activated DCs carrying skin-derived antigen also migrated from the skin to immune-inductive sites in gut mucosa and presented antigen directly to resident lymphocytes. These results and previous clinical trial results support the observation that TCI is a safe and effective strategy for inducing strong mucosal antibody and CTL responses.
Figures

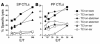


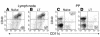

Similar articles
-
Transcutaneous immunization with a novel imiquimod nanoemulsion induces superior T cell responses and virus protection.J Dermatol Sci. 2017 Sep;87(3):252-259. doi: 10.1016/j.jdermsci.2017.06.012. Epub 2017 Jun 16. J Dermatol Sci. 2017. PMID: 28655469
-
Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge.Proc Natl Acad Sci U S A. 1998 Feb 17;95(4):1709-14. doi: 10.1073/pnas.95.4.1709. Proc Natl Acad Sci U S A. 1998. PMID: 9465081 Free PMC article.
-
[Dendritic cells of mucosa and skin: "recruited for vaccination"].Med Sci (Paris). 2007 Oct;23(10):819-25. doi: 10.1051/medsci/20072310819. Med Sci (Paris). 2007. PMID: 17937889 Review. French.
-
Mucosal immune responses induced by transcutaneous vaccines.Curr Top Microbiol Immunol. 2012;354:19-37. doi: 10.1007/82_2010_113. Curr Top Microbiol Immunol. 2012. PMID: 22435116 Review.
-
Transcutaneous immunization in mice: induction of T-helper and cytotoxic T lymphocyte responses and protection against human papillomavirus-induced tumors.Int J Cancer. 2006 Jan 15;118(2):364-72. doi: 10.1002/ijc.21360. Int J Cancer. 2006. PMID: 16052529
Cited by
-
Systemic immunization with CCL27/CTACK modulates immune responses at mucosal sites in mice and macaques.Vaccine. 2010 Feb 23;28(8):1942-51. doi: 10.1016/j.vaccine.2009.10.095. Vaccine. 2010. PMID: 20188250 Free PMC article.
-
Langerhans cells cross-present antigen derived from skin.Proc Natl Acad Sci U S A. 2006 May 16;103(20):7783-8. doi: 10.1073/pnas.0509307103. Epub 2006 May 3. Proc Natl Acad Sci U S A. 2006. PMID: 16672373 Free PMC article.
-
Non-Invasive Vaccines: Challenges in Formulation and Vaccine Adjuvants.Pharmaceutics. 2023 Aug 9;15(8):2114. doi: 10.3390/pharmaceutics15082114. Pharmaceutics. 2023. PMID: 37631328 Free PMC article. Review.
-
Antibody association with HER-2/neu-targeted vaccine enhances CD8 T cell responses in mice through Fc-mediated activation of DCs.J Clin Invest. 2008 May;118(5):1700-11. doi: 10.1172/JCI34333. J Clin Invest. 2008. PMID: 18398507 Free PMC article.
-
Immune cell trafficking: a novel perspective on the gut-skin axis.Inflamm Regen. 2024 Apr 24;44(1):21. doi: 10.1186/s41232-024-00334-5. Inflamm Regen. 2024. PMID: 38654394 Free PMC article. Review.
References
-
- Lehner T, et al. Protective mucosal immunity elicited by targeted iliac lymph node immunization with a subunit SIV envelope and core vaccine in macaques. Nat. Med. 1996;2:767–775. - PubMed
-
- Berzofsky JA, et al. Approaches to improve engineered vaccines for human immunodeficiency virus (HIV) and other viruses that cause chronic infections. Immunol. Rev. 1999;170:151–172. - PubMed
-
- Murphey-Corb M, et al. Selective induction of protective MHC class I restricted CTL in the intestinal lamina propria of rhesus monkeys by transient SIV infection of the colonic mucosa. J. Immunol. 1999;162:540–549. - PubMed
-
- Berzofsky JA, Ahlers JD, Belyakov IM. Strategies for designing and optimizing new generation vaccines. Nat. Rev. Immunol. 2001;1:209–219. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

