Anterior eye development and ocular mesenchyme: new insights from mouse models and human diseases
- PMID: 15057935
- PMCID: PMC2094210
- DOI: 10.1002/bies.20009
Anterior eye development and ocular mesenchyme: new insights from mouse models and human diseases
Abstract
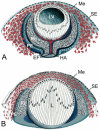
During development of the anterior eye segment, cells that originate from the surface epithelium or the neuroepithelium need to interact with mesenchymal cells, which predominantly originate from the neural crest. Failures of proper interaction result in a complex of developmental disorders such Peters' anomaly, Axenfeld-Rieger's syndrome or aniridia. Here we review the role of transcription factors that have been identified to be involved in the coordination of anterior eye development. Among these factors is PAX6, which is active in both epithelial and mesenchymal cells during ocular development, albeit at different doses and times. We propose that PAX6 is a key element that synchronizes the complex interaction of cell types of different origin, which are all needed for proper morphogenesis of the anterior eye. We discuss several molecular mechanisms that might explain the effects of haploinsufficiency of PAX6 and other transcription factors, and the broad variation of the resulting phenotypes.
Copyright 2004 Wiley Periodicals, Inc.
Figures




References
-
- Duke-Elder S, Cook C. In: Normal and Abnormal Development. Part 1. Embryology. Duke-Elder S, editor. Henry Kimpton; London: 1963.
-
- Haustein J. On the ultrastructure of the developing and adult mouse corneal stroma. Anat Embryol (Berl) 1983;168:291–305. - PubMed
-
- Pei YF, Rhodin JAG. The prenatal development of the mouse eye. Anat Rec. 1970;168:105–126. - PubMed
-
- Düblin I. Comparative embryologic studies of the early development of the cornea and the pupillary membrane in reptiles, birds and mammals. Acta Anat (Basel) 1970;76:381–408. - PubMed
-
- Kidson SH, Kume T, Deng K, Winfrey V, Hogan BL. The forkhead/winged-helix gene, Mf1, is necessary for the normal development of the cornea and formation of the anterior chamber in the mouse eye. Dev Biol. 1999;211:306–322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

