Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin
- PMID: 15059278
- PMCID: PMC400433
- DOI: 10.1186/ar1149
Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin
Abstract
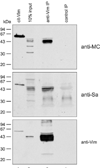
Antibodies directed to the Sa antigen are highly specific for rheumatoid arthritis (RA) and can be detected in approximately 40% of RA sera. The antigen, a doublet of protein bands of about 50 kDa, is present in placenta and in RA synovial tissue. Although it has been stated that the Sa antigen is citrullinated vimentin, experimental proof for this claim has never been published. In this study, we investigated the precise nature of the antigen. Peptide sequences that were obtained from highly purified Sa antigen were unique to vimentin. Recombinant vimentin, however, was not recognized by anti-Sa reference sera. In vivo, vimentin is subjected to various post-translational modifications, including citrullination. Since antibodies to citrullinated proteins are known to be highly specific for RA, we investigated whether Sa is citrullinated and found that Sa indeed is citrullinated vimentin. Anti-Sa antibodies thus belong to the family of anticitrullinated protein/peptide antibodies. The presence of the Sa antigen in RA synovial tissue, and the recent observation that vimentin is citrullinated in dying human macrophages, make citrullinated vimentin an interesting candidate autoantigen in RA and may provide new insights into the potential role of citrullinated synovial antigens and the antibodies directed to them in the pathophysiology of RA.
Figures



Comment in
-
Anti-Sa antibodies: prognostic and pathogenetic significance to rheumatoid arthritis.Arthritis Res Ther. 2004;6(2):86-9. doi: 10.1186/ar1171. Epub 2004 Mar 10. Arthritis Res Ther. 2004. PMID: 15059270 Free PMC article. Review.
References
-
- Mageed RA. The RF antigen. In: van Venrooij WJ, Maini RN, editor. In Manual of Biological Markers of Disease. Section B1.1. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 1–27.
-
- Lisse JR. Does rheumatoid factor always mean arthritis? Postgrad Med. 1993;94:133–134. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

