Ovariectomized rats as a model of postmenopausal osteoarthritis: validation and application
- PMID: 15059281
- PMCID: PMC400436
- DOI: 10.1186/ar1152
Ovariectomized rats as a model of postmenopausal osteoarthritis: validation and application
Abstract
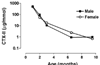
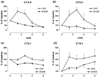
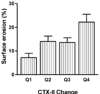
We aimed to assess the effect of ovariectomy on cartilage turnover and degradation, to evaluate whether ovariectomized (OVX) rats could form an experimental model of postmenopausal osteoarthritis. The effect of ovariectomy on cartilage was studied using two cohorts of female Sprague-Dawley rats, aged 5 and 7 months. In a third cohort, the effect of exogenous estrogen and a selective estrogen receptor modulator was analyzed. Knee joints were assessed by histological analysis of the articular cartilage after 9 weeks. Cartilage turnover was measured in urine by an immunoassay specific for collagen type II degradation products (CTX-II), and bone resorption was quantified in serum using an assay for bone collagen type I fragments (CTX-I). Surface erosion in the cartilage of the knee was more severe in OVX rats than in sham-operated animals, particularly in the 7-month-old cohort (P = 0.008). Ovariectomy also significant increased CTX-I and CTX-II. Both the absolute levels of CTX-II and the relative changes from baseline seen at week 4 correlated strongly with the severity of cartilage surface erosion at termination (r = 0.74, P < 0.01). Both estrogen and the selective estrogen receptor modulator inhibited the ovariectomy-induced acceleration of cartilage and bone turnover and significantly suppressed cartilage degradation and erosion seen in vehicle-treated OVX rats. The study indicates that estrogen deficiency accelerates cartilage turnover and increases cartilage surface erosion. OVX rats provide a useful experimental model for the evaluation of the chondroprotective effects of estrogens and estrogen-like substances and the model may be an in vivo representation of osteoarthritis in postmenopausal women.
Figures



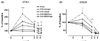
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

