pVHL modification by NEDD8 is required for fibronectin matrix assembly and suppression of tumor development
- PMID: 15060148
- PMCID: PMC381603
- DOI: 10.1128/MCB.24.8.3251-3261.2004
pVHL modification by NEDD8 is required for fibronectin matrix assembly and suppression of tumor development
Abstract
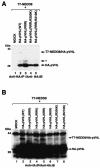
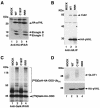
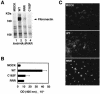
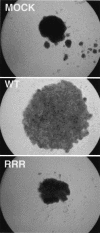
Functional inactivation of the von Hippel-Lindau (VHL) tumor suppressor gene is the cause of the familial VHL disease and most sporadic renal clear-cell carcinomas (RCC). pVHL has been shown to play a role in the destruction of hypoxia-inducible factor alpha (HIF-alpha) subunits via ubiquitin-mediated proteolysis and in the regulation of fibronectin matrix assembly. Although most disease-causing pVHL mutations hinder the regulation of the HIF pathway, every disease-causing pVHL mutant tested to date has failed to promote the assembly of the fibronectin matrix, underscoring its potential importance in VHL disease. Here, we report that a ubiquitin-like molecule called NEDD8 covalently modifies pVHL. A nonneddylateable pVHL mutant, while retaining its ability to ubiquitylate HIF, failed to bind to and promote the assembly of the fibronectin matrix. Expression of the neddylation-defective pVHL in RCC cells, while restoring the regulation of HIF, failed to promote the differentiated morphology in a three-dimensional growth assay and was insufficient to suppress the formation of tumors in SCID mice. These results suggest that NEDD8 modification of pVHL plays an important role in fibronectin matrix assembly and that in the absence of such regulation, an intact HIF pathway is insufficient to prevent VHL-associated tumorigenesis.
Figures

References
-
- Clifford, S. C., M. E. Cockman, A. C. Smallwood, D. R. Mole, E. R. Woodward, P. H. Maxwell, P. J. Ratcliffe, and E. R. Maher. 2001. Contrasting effects on HIF-1α regulation by disease-causing pVHL mutations correlate with patterns of tumourigenesis in von Hippel-Lindau disease. Hum. Mol. Genet. 10:1029-1038. - PubMed
-
- Cockman, M., N. Masson, D. Mole, P. Jaakkola, G. Chang, S. Clifford, E. Maher, C. Pugh, P. Ratcliffe, and P. Maxwell. 2000. Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J. Biol. Chem. 275:25733-25741. - PubMed
-
- Cohen, H., M. Zhou, A. Welsh, S. Zarghamee, H. Scholz, D. Mukhopadhyay, T. Kishida, B. Zbar, B. Knebelmann, and V. Sukhatme. 1999. An important von Hippel-Lindau tumor suppressor domain mediates Sp1-binding and self-association. Biochem. Biophys. Res. Commun. 266:43-50. - PubMed
-
- Epstein, A. C., J. M. Gleadle, L. A. McNeill, K. S. Hewitson, J. O'Rourke, D. R. Mole, M. Mukherji, E. Metzen, M. I. Wilson, A. Dhanda, Y. M. Tian, N. Masson, D. L. Hamilton, P. Jaakkola, R. Barstead, J. Hodgkin, P. H. Maxwell, C. W. Pugh, C. J. Schofield, and P. J. Ratcliffe. 2001. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107:43-54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
