Mutations linked to leukoencephalopathy with vanishing white matter impair the function of the eukaryotic initiation factor 2B complex in diverse ways
- PMID: 15060152
- PMCID: PMC381664
- DOI: 10.1128/MCB.24.8.3295-3306.2004
Mutations linked to leukoencephalopathy with vanishing white matter impair the function of the eukaryotic initiation factor 2B complex in diverse ways
Abstract
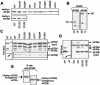
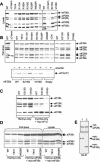
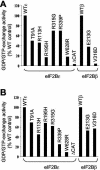
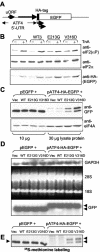
Leukoencephalopathy with vanishing white matter (VWM) is a severe inherited human neurodegenerative disorder that is caused by mutations in the genes for the subunits of eukaryotic initiation factor 2B (eIF2B), a heteropentameric guanine nucleotide exchange factor that regulates both global and mRNA-specific translation. Marked variability is evident in the clinical severity and time course of VWM in patients. Here we have studied the effects of VWM mutations on the function of human eIF2B. All the mutations tested cause partial loss of activity. Frameshift mutations in genes for eIF2Bepsilon or eIF2Bbeta lead to truncated polypeptides that fail to form complexes with the other subunits and are effectively null mutations. Certain point mutations also impair the ability of eIF2Bbeta or -epsilon to form eIF2B holocomplexes and also diminish the intrinsic nucleotide exchange activity of eIF2B. A point mutation in the catalytic domain of eIF2Bepsilon impairs its ability to bind the substrate, while two mutations in eIF2Bbeta actually enhance eIF2 binding. We provide evidence that expression of VWM mutant eIF2B may enhance the translation of specific mRNAs. The variability of the clinical phenotype in VWM may reflect the multiple ways in which VWM mutations affect eIF2B function.
Figures


References
-
- Clemens, M. J. 2001. Initiation factor eIF2α phosphorylation in stress responses and apoptosis. Prog. Mol. Subcell. Biol. 27:57-89. - PubMed
-
- Colthurst, D. R., D. G. Campbell, and C. G. Proud. 1987. Structure and regulation of eukaryotic initiation factor eIF-2. Sequence of the site in the alpha subunit phosphorylated by the haem-controlled repressor and by the double-stranded RNA-activated inhibitor. Eur. J. Biochem. 166:357-363. - PubMed
-
- Craddock, B. L., and C. G. Proud. 1996. The α-subunit of mammalian initiation factor eIF-2B is essential for catalytic activity. Biochem. Biophys. Res. Commun. 220:843-847. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
