Rescue of an hTERT mutant defective in telomere elongation by fusion with hPot1
- PMID: 15060173
- PMCID: PMC381596
- DOI: 10.1128/MCB.24.8.3552-3561.2004
Rescue of an hTERT mutant defective in telomere elongation by fusion with hPot1
Abstract
The protein hPot1 shares homology with telomere-binding proteins in lower eukaryotes and associates with single-stranded telomeric DNA in vitro as well as colocalizing with telomere-binding proteins in vivo. We now show that hPot1 is coimmunoprecipitated with telomeric DNA and that stable expression of this protein in telomerase-positive cells results in telomere elongation, supporting the idea that hPot1 is a bona fide mammalian telomere-binding protein. We previously found that mutations in the N-terminal DAT domain of the hTERT catalytic subunit of telomerase rendered the enzyme catalytically active but unable to elongate telomeres in vivo. This phenotype could be partially rescued by fusion with the double-stranded telomeric protein hTRF2. Given that hPot1 binds to single-stranded DNA in vitro (at the same site that hTERT binds to in vivo), we addressed whether fusion of hPot1 can rescue the DAT mutations more efficiently than that of hTRF2. We now report that a DAT mutant of hTERT is indeed efficiently rescued upon fusion to hPot1. However, this rescue depended on the ability of hPot1 to localize to telomeres rather than binding to DNA per se. These data support a model whereby the DAT domain of hTERT is implicated in telomere-telomerase associations.
Figures
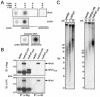
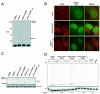


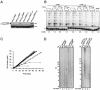
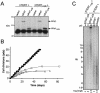
References
-
- Baumann, P., and T. R. Cech. 2001. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 292:1171-1175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
