Designed angiopoietin-1 variant, COMP-Ang1, protects against radiation-induced endothelial cell apoptosis
- PMID: 15060280
- PMCID: PMC397421
- DOI: 10.1073/pnas.0307575101
Designed angiopoietin-1 variant, COMP-Ang1, protects against radiation-induced endothelial cell apoptosis
Abstract
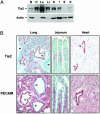
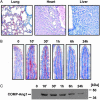
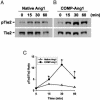
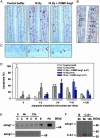
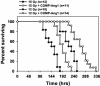
Radiation therapy is a widely used cancer treatment, but it causes side effects even when localized radiotherapy is used. Extensive apoptosis of microvascular endothelial cells of the lamina propria is the primary lesion initiating intestinal radiation damage after abdominal radiation therapy. Many in vitro studies suggest that angiopoietin-1 (Ang1) has potential therapeutic applications in enhancing endothelial cell survival. For in vivo use, we developed a soluble, stable, and potent Ang1 variant, COMP-Ang1. COMP-Ang1 is more potent than native Ang1 in phosphorylating the Tie2 receptor in lung endothelial cells in vivo. Interestingly, COMP-Ang1 administered i.v. was mainly localized to microvascular endothelial cells of the intestinal villi and lung but not to microvascular endothelial cells of the liver. In irradiated mice, i.v. COMP-Ang1 protected against radiation-induced apoptosis in microcapillary endothelial cells of the intestinal villi and prolonged survival. Thus, COMP-Ang1 could be used as a therapeutic protein for specific protection against endothelial cell injury.
Figures
References
-
- Paris, F., Fuks, Z., Kang, A., Capodieci, P., Juan, G., Ehleiter, D., Haimovitz-Friedman, A., Cordon-Cardo, C. & Kolesnick, R. (2001) Science 293, 293–297. - PubMed
-
- Okunieff, P., Mester, M., Wang, J., Maddox, T., Gong, X., Tang, D., Coffee, M. & Ding, I. (1998) Radiat. Res. 150, 204–211. - PubMed
-
- Veikkola, T. & Alitalo, K. (1999) Semin. Cancer Biol. 9, 211–220. - PubMed
-
- Davis, S., Aldrich, T. H., Jones, P. F., Acheson, A., Compton, D. L., Jain, V., Ryan, T. E., Bruno, J., Radziejewski, C., Maisonpierre, P. C., et al. (1996) Cell 87, 1161–1169. - PubMed
-
- Yancopoulos, G. D., Davis, S., Gale, N. W., Rudge, J. S., Wiegand, S. J. & Holash, J. (2000) Nature 407, 242–248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

