Pathophysiology of vascular dysfunction in a rat model of chronic joint inflammation
- PMID: 15064324
- PMCID: PMC1665086
- DOI: 10.1113/jphysiol.2004.062984
Pathophysiology of vascular dysfunction in a rat model of chronic joint inflammation
Abstract
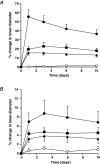
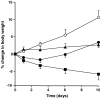
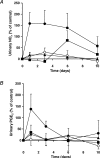
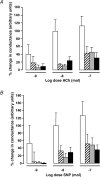
The impact of chronic joint inflammation on articular vascular function in rats was investigated to address whether joint swelling and the associated vascular dysfunction are dependent upon a common prostanoid mechanism. Urinary nitrate/nitrite (NO(x)) and PGE(2) excretion, knee joint diameter and body weight were measured following induction of adjuvant-induced arthritis (AIA). Ten days postinduction of AIA, joint vascular reactivity was assessed by measuring the perfusion response using a laser Doppler imager (LDI) to topical application of acetylcholine (ACh) and sodium nitroprusside (SNP). Four groups were compared: a non-inflamed control group and three AIA groups treated i.p. with vehicle, indomethacin or SC-236 (at equimolar doses). The selective cyclooxygenase-2 (COX-2) inhibitor (SC-236) was used to differentiate between COX-1 and -2-derived prostaglandins. Urinary NO(x) and PGE(2) levels increased substantially during the early phase of AIA but decreased thereafter. Toxicity to indomethacin but not SC-236 was observed, as indicated by a marked decrease in body weight. Joint swelling was similarly attenuated by indomethacin and SC-236 (P= 0.0001 cf. vehicle-treated AIA; n= 5-6 per group), indicating that this is due to COX-2 and not COX-1 inhibition. The AIA-induced changes in urinary NO(x) and PGE(2) were corrected by both COX inhibitors. While vascular reactivity to ACh and SNP was significantly attenuated by AIA (P < 0.002; n= 5-10 per group), the perfusion responses to these vasodilating agents were similar in all three AIA groups, demonstrating that the vascular dysfunction was not corrected by inhibition of either COX-1 or COX-2 enzymes. Furthermore, the attenuation of both ACh and SNP-induced responses in AIA suggest that vascular dysfunction was not exclusively endothelial in nature. In conclusion, the joint swelling and vascular dysfunction associated with AIA appear to be mediated, at least in part, by independent mechanisms. While COX-1/COX-2 inhibition reduced joint swelling, vascular dysfunction in AIA is independent of constitutive or inducible prostanoid mechanisms, and appears not to be solely endothelial-derived, but to involve other components such as the vascular smooth muscle.
Figures
Similar articles
-
Expression of constitutive but not inducible cyclooxygenase maintains articular perfusion in the rat knee.Exp Physiol. 2001 Mar;86(2):191-7. doi: 10.1113/eph8602129. Exp Physiol. 2001. PMID: 11429634
-
Pathophysiological basis of acute inflammatory hyperaemia in the rat knee: roles of cyclo-oxygenase-1 and -2.J Physiol. 2002 Mar 1;539(Pt 2):579-87. doi: 10.1113/jphysiol.2001.013473. J Physiol. 2002. PMID: 11882689 Free PMC article.
-
Involvement of cyclooxygenase-derived prostaglandin E2 and nitric oxide in the protection of rat pancreas afforded by low dose of lipopolysaccharide.J Physiol Pharmacol. 2001 Mar;52(1):107-26. J Physiol Pharmacol. 2001. PMID: 11321505
-
Characterization of the induction of nitric oxide synthase and cyclo-oxygenase in rat aorta in organ culture.Br J Pharmacol. 1997 May;121(1):125-33. doi: 10.1038/sj.bjp.0701100. Br J Pharmacol. 1997. PMID: 9146896 Free PMC article.
-
Dual acting anti-inflammatory drugs: a reappraisal.Pharmacol Res. 2001 Dec;44(6):437-50. doi: 10.1006/phrs.2001.0872. Pharmacol Res. 2001. PMID: 11735348 Review.
Cited by
-
Hydrogen peroxide mediates pro-inflammatory cell-to-cell signaling: a new therapeutic target for inflammation?Neural Regen Res. 2019 Aug;14(8):1430-1437. doi: 10.4103/1673-5374.253529. Neural Regen Res. 2019. PMID: 30964069 Free PMC article.
-
Total sleep deprivation alters endothelial function in rats: a nonsympathetic mechanism.Sleep. 2014 Mar 1;37(3):465-73. doi: 10.5665/sleep.3476. Sleep. 2014. PMID: 24587568 Free PMC article.
-
Hippocampal neuronal metal ion imbalance related oxidative stress in a rat model of chronic aluminum exposure and neuroprotection of meloxicam.Behav Brain Funct. 2014 Mar 11;10:6. doi: 10.1186/1744-9081-10-6. Behav Brain Funct. 2014. PMID: 24618126 Free PMC article.
-
Cartilage degeneration is associated with augmented chemically-induced joint pain in rats: a pilot study.Clin Orthop Relat Res. 2010 May;468(5):1423-7. doi: 10.1007/s11999-009-1193-z. Epub 2009 Dec 15. Clin Orthop Relat Res. 2010. PMID: 20013164 Free PMC article.
-
Treatment with the arginase inhibitor Nw-hydroxy-nor-L-arginine restores endothelial function in rat adjuvant-induced arthritis.Arthritis Res Ther. 2012 May 30;14(3):R130. doi: 10.1186/ar3860. Arthritis Res Ther. 2012. PMID: 22647483 Free PMC article.
References
-
- Bileviciute I, Lundeberg T, Ekblom A, Theodorsson E. Bilateral changes of substance P-, neurokinin A- and neuropeptides Y-like immunoreactivity in rat knee joint synovial fluid during acute monoarthritis. Neurosci Lett. 1993;153:37–40. - PubMed
-
- Carpenter MA, Everett LD, Hall MA. High-resolution magnetic resonance imaging of arthritic pathology in the rat knee. Skeletal Radiol. 1994;23:429–437. - PubMed
-
- Dela Torre A, Schroeder RA, Kuo PC. Alteration of NF-kappa B P50 binding kinetics by S-nitrosylation. Biochem Biophys Res Commun. 1997;238:703–706. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

