Ectopic expression of maize polyamine oxidase and pea copper amine oxidase in the cell wall of tobacco plants
- PMID: 15064377
- PMCID: PMC419818
- DOI: 10.1104/pp.103.036764
Ectopic expression of maize polyamine oxidase and pea copper amine oxidase in the cell wall of tobacco plants
Abstract
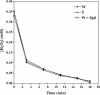
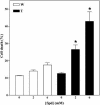
To test the feasibility of altering polyamine levels by influencing their catabolic pathway, we obtained transgenic tobacco (Nicotiana tabacum) plants constitutively expressing either maize (Zea mays) polyamine oxidase (MPAO) or pea (Pisum sativum) copper amine oxidase (PCuAO), two extracellular and H(2)O(2)-producing enzymes. Despite the high expression levels of the transgenes in the extracellular space, the amount of free polyamines in the homozygous transgenic plants was similar to that in the wild-type ones, suggesting either a tight regulation of polyamine levels or a different compartmentalization of the two recombinant proteins and the bulk amount of endogenous polyamines. Furthermore, no change in lignification levels and plant morphology was observed in the transgenic plants compared to untransformed plants, while a small but significant change in reactive oxygen species-scavenging capacity was verified. Both the MPAO and the PCuAO tobacco transgenic plants produced high amounts of H(2)O(2) only in the presence of exogenously added enzyme substrates. These observations provided evidence for the limiting amount of freely available polyamines in the extracellular space in tobacco plants under physiological conditions, which was further confirmed for untransformed maize and pea plants. The amount of H(2)O(2) produced by exogenously added polyamines in cell suspensions from the MPAO transgenic plants was sufficient to induce programmed cell death, which was sensitive to catalase treatment and required gene expression and caspase-like activity. The MPAO and PCuAO transgenic plants represent excellent tools to study polyamine secretion and conjugation in the extracellular space, as well as to determine when and how polyamine catabolism actually intervenes both in cell wall development and in response to stress.
Figures

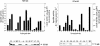





References
-
- Biondi S, Scaramagli S, Capitani F, Altamura MM, Torrigiani P (2001) Methyl jasmonate upregulates biosynthetic gene expression, oxidation and conjugation of polyamines, and inhibits shoot formation in tobacco thin layers. J Exp Bot 52: 231–242 - PubMed
-
- Bouchereau A, Aziz A, Larher F, Martin–Tanguy J (1999) Polyamines and environmental challenges: recent development. Plant Sci 140: 103–125
-
- Capell T, Bassie L, Topsom L, Hitchin E, Christou P (2000) Simultaneous reduction of the activity of the two related enzymes involved in early steps of the polyamine biosynthetic pathway by a single antisense cDNA in transgenic rice. Mol Gen Genet 264: 470–476 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

