Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure
- PMID: 15064385
- PMCID: PMC419829
- DOI: 10.1104/pp.103.032250
Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure
Abstract
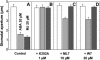
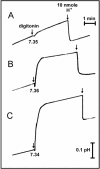
Signaling events during abscisic acid (ABA) or methyl jasmonate (MJ)-induced stomatal closure were examined in Arabidopsis wild type, ABA-insensitive (ost1-2), and MJ-insensitive mutants (jar1-1) in order to examine a crosstalk between ABA and MJ signal transduction. Some of the experiments were performed on epidermal strips of Pisum sativum. Stomata of jar1-1 mutant plants are insensitive to MJ but are able to close in response to ABA. However, their sensitivity to ABA is less than that of wild-type plants. Reciprocally, the stomata of ost1-2 are insensitive to ABA but are able to close in response to MJ to a lesser extent compared to wild-type plants. Both MJ and ABA promote H(2)O(2) production in wild-type guard cells, while exogenous application of diphenylene iodonium (DPI) chloride, an inhibitor of NAD(P)H oxidases, results in the suppression of ABA- and MJ-induced stomatal closure. ABA elevates H(2)O(2) production in wild-type and jar1-1 guard cells but not in ost1-2, whereas MJ induces H(2)O(2) production in both wild-type and ost1-2 guard cells, but not in jar1-1. MJ-induced stomatal closing is suppressed in the NAD(P)H oxidase double mutant atrbohD/F and in the outward potassium channel mutant gork1. Furthermore, MJ induces alkalization in guard cell cytosol, and MJ-induced stomatal closing is inhibited by butyrate. Analyses of the kinetics of cytosolic pH changes and reactive oxygen species (ROS) production show that the alkalization of cytoplasm precedes ROS production during the stomatal response to both ABA and MJ. Our results further indicate that JAR1, as OST1, functions upstream of ROS produced by NAD(P)H oxidases and that the cytoplasmic alkalization precedes ROS production during MJ or ABA signal transduction in guard cells.
Figures


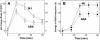
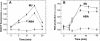
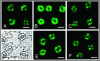
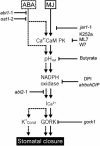
References
-
- Ache P, Becker D, Ivashikina N, Dietrich P, Roelfsema MR, Hedrich R (2000) GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K+-selective, K+-sensing ion channel. FEBS Lett 486: 93–98 - PubMed
-
- Agrios GN (1997) Plant Pathology, Ed 4. Academic Press, San Diego, pp 46–52
-
- Allen GJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JF (2000) Alternation of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289: 2338–2342 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

