Modulation of energy-dependent quenching of excitons in antennae of higher plants
- PMID: 15064404
- PMCID: PMC397417
- DOI: 10.1073/pnas.0401269101
Modulation of energy-dependent quenching of excitons in antennae of higher plants
Abstract
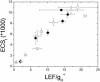
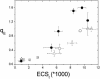
Energy-dependent exciton quenching, or q(E), protects the higher plant photosynthetic apparatus from photodamage. Initiation of q(E) involves protonation of violaxanthin deepoxidase and PsbS, a component of the photosystem II antenna complex, as a result of lumen acidification driven by photosynthetic electron transfer. It has become clear that the response of q(E) to linear electron flow, termed "q(E) sensitivity," must be modulated in response to fluctuating environmental conditions. Previously, three mechanisms have been proposed to account for q(E) modulation: (i) the sensitivity of q(E) to the lumen pH is altered; (ii) elevated cyclic electron flow around photosystem I increases proton translocation into the lumen; and (iii) lowering the conductivity of the thylakoid ATP synthase to protons (g(H+)) allows formation of a larger steady-state proton motive force (pmf). Kinetic analysis of the electrochromic shift of intrinsic thylakoid pigments, a linear indicator of transthylakoid electric field component, suggests that, when CO(2) alone was lowered from 350 ppm to 50 ppm CO(2), modulation of q(E) sensitivity could be explained solely by changes in conductivity. Lowering both CO(2) (to 50 ppm) and O(2) (to 1%) resulted in an additional increase in q(E) sensitivity that could not be explained by changes in conductivity or cyclic electron flow associated with photosystem I. Evidence is presented for a fourth mechanism, in which changes in q(E) sensitivity result from variable partitioning of proton motive force into the electric field and pH gradient components. The implications of this mechanism for the storage of proton motive force and the regulation of the light reactions are discussed.
Figures


References
-
- Ort, D. R. & Yocum, C. F. (1996) in Oxygenic Photosynthesis: The Light Reactions, eds. Ort, D. R. & Yocum, C. F. (Kluwer, Dordrecht, The Netherlands), pp. 1–9.
-
- Allen, J. F. (2002) Cell 110, 273–276. - PubMed
-
- Capaldi, R. A. & Aggeler, R. (2002) Trends Biochem. Sci. 27, 154–160. - PubMed
-
- Pfannschmidt, T. (2003) Trends Plant Sci. 8, 33–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

