Phosphorylation and ubiquitination of oncogenic mutants of beta-catenin containing substitutions at Asp32
- PMID: 15064718
- PMCID: PMC2267883
- DOI: 10.1038/sj.onc.1207634
Phosphorylation and ubiquitination of oncogenic mutants of beta-catenin containing substitutions at Asp32
Abstract
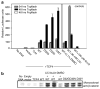
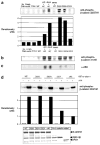
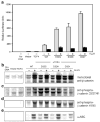
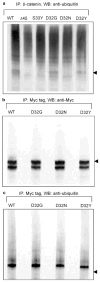
Beta-Catenin, a member of the Wnt signaling pathway, is downregulated by glycogen synthase kinase-3beta (GSK-3beta)-dependent phosphorylation of Ser/Thr residues in the N-terminus of the protein, followed by ubiquitination and proteosomal degradation. In human and rodent cancers, mutations that substitute one of the critical Ser/Thr residues in the GSK-3beta region of beta-catenin stabilize the protein and activate beta-catenin/TCF/LEF target genes. This study examined three oncogenic beta-catenin mutants from rat colon tumors containing substitutions adjacent to amino-acid residue Ser33, a key target for phosphorylation by GSK-3beta. Compared with wild-type beta-catenin (WT), the beta-catenin mutants D32G, D32N, and D32Y strongly activated TCF-4-dependent transcription in HEK293 cells, and there was accumulation of beta-catenin in the cell lysates. Immunoblotting with phosphospecific antibodies indicated that there was little if any effect on the phosphorylation of Ser37, Thr41 or Ser45; however, the phosphorylation of Ser33 appeared to be affected in the beta-catenin mutants. Specifically, antiphospho-beta-catenin 33/37/41 antibody identified high, intermediate and low expression levels of phosphorylated beta-catenin in cells transfected with D32G, D32N and D32Y, respectively. Experiments with the proteosome inhibitor N-acetyl-Leu-Leu-norleucinal (ALLN) revealed ubiquitinated bands on all three mutant beta-catenins, as well as on WT beta-catenin. The relative order of ubiquitination was WT>D32G>D32N>D32Y, in parallel with findings from the phosphorylation studies. These results are discussed in the context of previous studies, which indicated that amino-acid residue D32 lies within the ubiquitination recognition motif of beta-catenin.
Copyright 2004 Nature Publishing Group
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

