Genetic evidence that the Vibrio cholerae monolayer is a distinct stage in biofilm development
- PMID: 15066042
- PMCID: PMC2501105
- DOI: 10.1111/j.1365-2958.2004.04000.x
Genetic evidence that the Vibrio cholerae monolayer is a distinct stage in biofilm development
Abstract
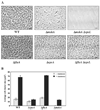
Biofilm development is conceived as a developmental process in which free swimming cells attach to a surface, first transiently and then permanently, as a single layer. This monolayer of immobilized cells gives rise to larger cell clusters that eventually develop into the biofilm, a three-dimensional structure consisting of large pillars of bacteria interspersed with water channels. Previous studies have shown that efficient development of the Vibrio cholerae biofilm requires a combination of pili, flagella and exopolysaccharide. Little is known, however, regarding the requirements for monolayer formation by wild-type V. cholerae. In this work, we have isolated the wild-type V. cholerae monolayer and demonstrated that the environmental signals, bacterial structures, and transcription profiles that induce and stabilize the monolayer state are unique. Cells in a monolayer are specialized to maintain their attachment to a surface. The surface itself activates mannose-sensitive haemagglutinin type IV pilus (MSHA)-mediated attachment, which is accompanied by repression of flagellar gene transcription. In contrast, cells in a biofilm are specialized to maintain intercellular contacts. Progression to this stage occurs when exopolysaccharide synthesis is induced by environmental monosaccharides. We propose a model for biofilm development in natural environments in which cells form a stable monolayer on a surface. As biotic surfaces are degraded with subsequent release of carbohydrates, the monolayer develops into a biofilm.
Figures





References
-
- Allison DG, Ruiz B, SanJose C, Jaspe A, Gilbert P. Extracellular products as mediators of the formation and detachment of Pseudomonas fluorescens bio-films. FEMS Microbiol Lett. 1998;167:179–184. - PubMed
-
- Bechet M, Blondeau R. Factors associated with the adherence and biofilm formation by Aeromonas caviae on glass surfaces. J Appl Microbiol. 2003;94:1072–1078. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

