Molecular basis for anaerobic growth of Saccharomyces cerevisiae on xylose, investigated by global gene expression and metabolic flux analysis
- PMID: 15066826
- PMCID: PMC383160
- DOI: 10.1128/AEM.70.4.2307-2317.2004
Molecular basis for anaerobic growth of Saccharomyces cerevisiae on xylose, investigated by global gene expression and metabolic flux analysis
Abstract
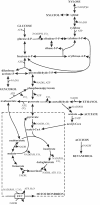
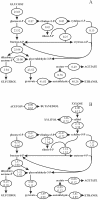
Yeast xylose metabolism is generally considered to be restricted to respirative conditions because the two-step oxidoreductase reactions from xylose to xylulose impose an anaerobic redox imbalance. We have recently developed, however, a Saccharomyces cerevisiae strain that is at present the only known yeast capable of anaerobic growth on xylose alone. Using transcriptome analysis of aerobic chemostat cultures grown on xylose-glucose mixtures and xylose alone, as well as a combination of global gene expression and metabolic flux analysis of anaerobic chemostat cultures grown on xylose-glucose mixtures, we identified the distinguishing characteristics of this unique phenotype. First, the transcript levels and metabolic fluxes throughout central carbon metabolism were significantly higher than those in the parent strain, and they were most pronounced in the xylose-specific, pentose phosphate, and glycerol pathways. Second, differential expression of many genes involved in redox metabolism indicates that increased cytosolic NADPH formation and NADH consumption enable a higher flux through the two-step oxidoreductase reaction of xylose to xylulose in the mutant. Redox balancing is apparently still a problem in this strain, since anaerobic growth on xylose could be improved further by providing acetoin as an external NADH sink. This improved growth was accompanied by an increased ATP production rate and was not accompanied by higher rates of xylose uptake or cytosolic NADPH production. We concluded that anaerobic growth of the yeast on xylose is ultimately limited by the rate of ATP production and not by the redox balance per se, although the redox imbalance, in turn, limits ATP production.
Figures

References
-
- Andreasen, A. A., and T. J. B. Stier. 1953. Anaerobic nutrition of Saccharomyces cerevisiae. 1. Ergosterol requirement for growth in a defined medium. J. Cell. Comp. Physiol. 41:23-36. - PubMed
-
- Bakker, B. M., K. M. Overkamp, A. J. van Maris, P. Kötter, M. A. Luttik, J. P. van Dijken, and J. T. Pronk. 2001. Stoichiometry and compartmentation of NADH metabolism in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 25:15-37. - PubMed
-
- Boer, V. M., J. H. de Winde, J. T. Pronk, and M. D. Piper. 2003. The genome-wide transcriptional responses of Saccharomyces cerevisiae grown on glucose in aerobic chemostat cultures limited for carbon, nitrogen, phosphorus, or sulfur. J. Biol. Chem. 278:3265-3274. - PubMed
-
- Boles, E., W. Lehnert, and F. K. Zimmermann. 1993. The role of the NAD-dependent glutamate dehydrogenase in restoring growth on glucose of a Saccharomyces cerevisiae phosphoglucose isomerase mutant. Eur. J. Biochem. 217:469-477. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

