Bradykinin-induced relaxation of coronary microarteries: S-nitrosothiols as EDHF?
- PMID: 15066907
- PMCID: PMC1574930
- DOI: 10.1038/sj.bjp.0705747
Bradykinin-induced relaxation of coronary microarteries: S-nitrosothiols as EDHF?
Abstract
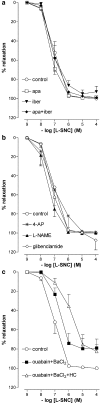
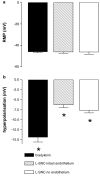
1. To investigate whether S-nitrosothiols, in addition to NO, mediate bradykinin-induced vasorelaxation, porcine coronary microarteries (PCMAs) were mounted in myographs. 2. Following preconstriction, concentration-response curves (CRCs) were constructed to bradykinin, the NO donors S-nitroso-N-penicillamine (SNAP) and diethylamine NONOate (DEA-NONOate) and the S-nitrosothiols L-S-nitrosocysteine (L-SNC) and D-SNC. All agonists relaxed PCMAs. L-SNC was approximately 5-fold more potent than D-SNC. 3. The guanylyl cyclase inhibitor ODQ and the NO scavenger hydroxocobalamin induced a larger shift of the bradykinin CRC than the NO synthase inhibitor L-NAME, although all three inhibitors equally suppressed bradykinin-induced cGMP responses. 4. Complete blockade of bradykinin-induced relaxation was obtained with L-NAME in the presence of the large- and intermediate-conductance Ca(2+)-activated K(+)-channel (BK(Ca), IK(Ca)) blocker charybdotoxin and the small-conductance Ca(2+)-activated K(+)-channel (SK(Ca)) channel blocker apamin, but not in the presence of L-NAME, apamin and the BK(Ca) channel blocker iberiotoxin. 5. Inhibitors of cytochrome P450 epoxygenase, cyclooxygenase, voltage-dependent K(+) channels and ATP-sensitive K(+) channels did not affect bradykinin-induced relaxation. 6. SNAP-, DEA-NONOate- and D-SNC-induced relaxations were mediated entirely by the NO-guanylyl cyclase pathway. L-SNC-induced relaxations were partially blocked by charybdotoxin+apamin, but not by iberiotoxin+apamin, and this blockade was abolished following endothelium removal. ODQ, but not hydroxocobalamin, prevented L-SNC-induced increases in cGMP, and both drugs shifted the L-SNC CRC 5-10-fold to the right. 7. L-SNC hyperpolarized intact and endothelium-denuded coronary arteries. 8. Our results support the concept that bradykinin-induced relaxation is mediated via de novo synthesized NO and a non-NO, endothelium-derived hyperpolarizing factor (EDHF). S-nitrosothiols, via stereoselective activation of endothelial IK(Ca) and SK(Ca) channels, and through direct effects on smooth muscle cells, may function as an EDHF in porcine coronary microarteries.
Figures
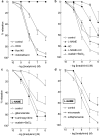
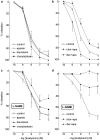


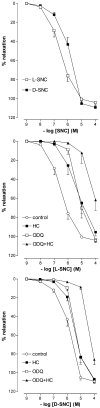
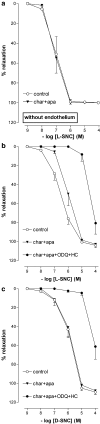
Similar articles
-
L-S-nitrosothiols: endothelium-derived hyperpolarizing factors in porcine coronary arteries?J Hypertens. 2004 Oct;22(10):1927-36. doi: 10.1097/00004872-200410000-00015. J Hypertens. 2004. PMID: 15361764
-
Contribution of K+ channels and ouabain-sensitive mechanisms to the endothelium-dependent relaxations of horse penile small arteries.Br J Pharmacol. 1998 Apr;123(8):1609-20. doi: 10.1038/sj.bjp.0701780. Br J Pharmacol. 1998. PMID: 9605568 Free PMC article.
-
Light-induced vs. bradykinin-induced relaxation of coronary arteries: do S-nitrosothiols act as endothelium-derived hyperpolarizing factors?J Hypertens. 2009 Aug;27(8):1631-40. doi: 10.1097/HJH.0b013e32832bff54. J Hypertens. 2009. PMID: 19421072
-
EDHF: an update.Clin Sci (Lond). 2009 Jul 16;117(4):139-55. doi: 10.1042/CS20090096. Clin Sci (Lond). 2009. PMID: 19601928 Review.
-
Endothelial potassium channels, endothelium-dependent hyperpolarization and the regulation of vascular tone in health and disease.Clin Exp Pharmacol Physiol. 2004 Sep;31(9):641-9. doi: 10.1111/j.1440-1681.2004.04053.x. Clin Exp Pharmacol Physiol. 2004. PMID: 15479173 Review.
Cited by
-
Phosphodiesterase-5 activity exerts a coronary vasoconstrictor influence in awake swine that is mediated in part via an increase in endothelin production.Am J Physiol Heart Circ Physiol. 2014 Mar;306(6):H918-27. doi: 10.1152/ajpheart.00331.2013. Epub 2014 Jan 24. Am J Physiol Heart Circ Physiol. 2014. PMID: 24464751 Free PMC article.
-
Local and systemic vasodilatory effects of low molecular weight S-nitrosothiols.Free Radic Biol Med. 2016 Feb;91:215-23. doi: 10.1016/j.freeradbiomed.2015.12.009. Epub 2015 Dec 12. Free Radic Biol Med. 2016. PMID: 26686469 Free PMC article.
-
S-nitrosothiols dilate the mesenteric artery more potently than the femoral artery by a cGMP and L-type calcium channel-dependent mechanism.Nitric Oxide. 2016 Aug 31;58:20-7. doi: 10.1016/j.niox.2016.05.006. Epub 2016 May 25. Nitric Oxide. 2016. PMID: 27235767 Free PMC article.
-
An Update on Thiol Signaling: S-Nitrosothiols, Hydrogen Sulfide and a Putative Role for Thionitrous Acid.Antioxidants (Basel). 2020 Mar 10;9(3):225. doi: 10.3390/antiox9030225. Antioxidants (Basel). 2020. PMID: 32164188 Free PMC article. Review.
-
Regulation of Coronary Blood Flow.Compr Physiol. 2017 Mar 16;7(2):321-382. doi: 10.1002/cphy.c160016. Compr Physiol. 2017. PMID: 28333376 Free PMC article. Review.
References
-
- ARCHER S.L., GRAGASIN F.S., WU X., WANG S., MCMURTRY S., KIM D.H., PLATONOV M., KOSHAL A., HASHIMOTO K., CAMPBELL W.B., FALCK J.R., MICHELAKIS E.D. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BK(Ca) channels. Circulation. 2003;107:769–776. - PubMed
-
- BOLOTINA V.M., NAJIBI S., PALACINO J.J., PAGANO P.J., COHEN R.A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. - PubMed
-
- BUSSE R., EDWARDS G., FELETOU M., FLEMING I., VANHOUTTE P.M., WESTON A.H. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

