In vivo molecular imaging of acute and subacute thrombosis using a fibrin-binding magnetic resonance imaging contrast agent
- PMID: 15066940
- PMCID: PMC2910574
- DOI: 10.1161/01.CIR.0000127034.50006.C0
In vivo molecular imaging of acute and subacute thrombosis using a fibrin-binding magnetic resonance imaging contrast agent
Abstract
Background: Plaque rupture with subsequent thrombosis is recognized as the underlying pathophysiology of most acute coronary syndromes and stroke. Thus, direct thrombus visualization may be beneficial for both diagnosis and guidance of therapy. We sought to test the feasibility of direct imaging of acute and subacute thrombosis using MRI together with a novel fibrin-binding gadolinium-labeled peptide, EP-1873, in an experimental animal model of plaque rupture and thrombosis.
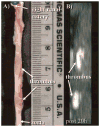
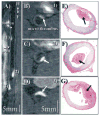
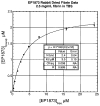
Methods and results: Fifteen male New Zealand White rabbits (weight, approximately 3.5 kg) were made atherosclerotic by feeding a high-cholesterol diet after endothelial aortic injury. Plaque rupture was then induced with the use of Russell's viper venom (RVV) and histamine. Subsequently, MRI of the subrenal aorta was performed before RVV, after RVV, and after EP-1873. Histology was performed on regions suggested by MRI to contain thrombus. Nine rabbits (60%) developed plaque rupture and thrombus, including 25 thrombi visually apparent on MRI as "hot spots" after injection of EP-1873. Histological correlation confirmed all 25 thrombi (100%), with no thrombi seen in the other regions of the aorta. In the remaining 6 rabbits (control) without plaque rupture, no thrombus was observed on the MR images or on histology.
Conclusions: We demonstrate the feasibility of in vivo "molecular" MRI for the detection of acute and subacute thrombosis using a novel fibrin-binding MRI contrast agent in an animal model of atherosclerosis and acute/subacute thrombosis. Potential clinical applications include thrombus detection in acute coronary syndromes and stroke.
Figures




References
-
- Fuster V, Badimon L, Badimon JJ, et al. The pathogenesis of coronary artery disease and the acute coronary syndromes. N Engl J Med. 1992;326:242–250. - PubMed
-
- Grotta JC. Adding to the effectiveness of intravenous tissue plasminogen activator for treating acute stroke. Circulation. 2003;107:2769–2770. - PubMed
-
- de Korte CL, Sierevogel MJ, Mastik F, et al. Identification of atherosclerotic plaque components with intravascular ultrasound elastography in vivo: a Yucatan pig study. Circulation. 2002;105:1627–1630. - PubMed
-
- Abela GS, Eisenberg JD, Mittleman MA, et al. Detecting and differentiating white from red coronary thrombus by angiography in angina pectoris and in acute myocardial infarction. Am J Cardiol. 1999;83:94–97. A8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

