Asymmetric autocatalysis and its implications for the origin of homochirality
- PMID: 15067112
- PMCID: PMC395976
- DOI: 10.1073/pnas.0308363101
Asymmetric autocatalysis and its implications for the origin of homochirality
Abstract
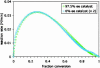
An autocatalytic reaction in which the reaction product serves as a catalyst to produce more of itself and to suppress production of its enantiomer serves as a mechanistic model for the evolution of homochirality. The Soai reaction provided experimental confirmation of this concept, nearly 50 years after it was first proposed. This Perspective offers a rationalization of the Soai autocatalytic reaction; accounting for enantiomeric excess and rate observations, that is both simple as well as gratifying in its implications for the chemical origin of life.
Figures
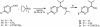

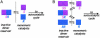


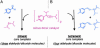
References
-
- Soai, K., Shibata, T., Morioka, H. & Choji, K. (1995) Nature 378, 767–768.
-
- Shibata, T., Choji, K., Hayase, T., Aizu, Y. & Soai, K. (1996) Chem. Commun. 1235–1236.
-
- Shibata, T., Morioka, H., Hayase, T., Choji, K. & Soai, K. (1996) J. Am. Chem. Soc. 118, 471–472.
-
- Soai, K., Niwa, S. & Hori, H. (1990) J. Chem. Soc. Chem. Commun. 1990, 982–983.
-
- Frank, F. C. (1953) Biochim. Biophys. Acta 11, 459–463. - PubMed
LinkOut - more resources
Full Text Sources

