Blockade of T cell costimulation reveals interrelated actions of CD4+ and CD8+ T cells in control of SIV replication
- PMID: 15067316
- PMCID: PMC362114
- DOI: 10.1172/JCI19442
Blockade of T cell costimulation reveals interrelated actions of CD4+ and CD8+ T cells in control of SIV replication
Abstract
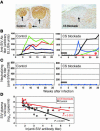
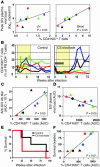
In vivo blockade of CD28 and CD40 T cell costimulation pathways during acute simian immunodeficiency virus (SIV) infection of rhesus macaques was performed to assess the relative contributions of CD4+ T cells, CD8+ T cells, and Ab responses in modulating SIV replication and disease progression. Transient administration of CTLA4-Ig and anti-CD40L mAb to SIV-infected rhesus macaques resulted in dramatic inhibition of the generation of both SIV-specific cellular and humoral immune responses. Acute levels of proliferating CD8+ T cells were associated with early control of SIV viremia but did not predict ensuing set point viremia or survival. The level of in vivo CD4+ T cell proliferation during acute SIV infection correlated with concomitant peak levels of SIV plasma viremia, whereas measures of in vivo CD4+ T cell proliferation that extended into chronic infection correlated with lower SIV viral load and increased survival. These results suggest that proliferating CD4+ T cells function both as sources of virus production and as antiviral effectors and that increased levels of CD4+ T cell proliferation during SIV infections reflect antigen-driven antiviral responses rather than a compensatory homeostatic response. These results highlight the interrelated actions of CD4+ and CD8+ T cell responses in vivo that modulate SIV replication and pathogenesis.
Figures




Comment in
-
The immune response to AIDS virus infection: good, bad, or both?J Clin Invest. 2004 Mar;113(6):808-10. doi: 10.1172/JCI21318. J Clin Invest. 2004. PMID: 15067312 Free PMC article.
References
-
- Kuroda MJ, et al. Emergence of CTL coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J. Immunol. 1999;162:5127–5133. - PubMed
-
- Mellors JW, et al. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

