Endothelin-1 regulates cardiac sympathetic innervation in the rodent heart by controlling nerve growth factor expression
- PMID: 15067320
- PMCID: PMC362115
- DOI: 10.1172/JCI19480
Endothelin-1 regulates cardiac sympathetic innervation in the rodent heart by controlling nerve growth factor expression
Abstract
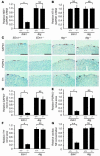
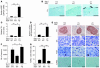
The cardiac sympathetic nerve plays an important role in regulating cardiac function, and nerve growth factor (NGF) contributes to its development and maintenance. However, little is known about the molecular mechanisms that regulate NGF expression and sympathetic innervation of the heart. In an effort to identify regulators of NGF in cardiomyocytes, we found that endothelin-1 specifically upregulated NGF expression in primary cultured cardiomyocytes. Endothelin-1-induced NGF augmentation was mediated by the endothelin-A receptor, Gibetagamma, PKC, the Src family, EGFR, extracellular signal-regulated kinase, p38MAPK, activator protein-1, and the CCAAT/enhancer-binding protein delta element. Either conditioned medium or coculture with endothelin-1-stimulated cardiomyocytes caused NGF-mediated PC12 cell differentiation. NGF expression, cardiac sympathetic innervation, and norepinephrine concentration were specifically reduced in endothelin-1-deficient mouse hearts, but not in angiotensinogen-deficient mice. In endothelin-1-deficient mice the sympathetic stellate ganglia exhibited excess apoptosis and displayed loss of neurons at the late embryonic stage. Furthermore, cardiac-specific overexpression of NGF in endothelin-1-deficient mice overcame the reduced sympathetic innervation and loss of stellate ganglia neurons. These findings indicate that endothelin-1 regulates NGF expression in cardiomyocytes and plays a critical role in sympathetic innervation of the heart.
Figures
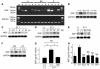

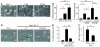

Comment in
-
Sculpting organ innervation.J Clin Invest. 2004 Mar;113(6):811-3. doi: 10.1172/JCI21309. J Clin Invest. 2004. PMID: 15067313 Free PMC article.
References
-
- Loring JF, Erickson CA. Neural crest cell migratory pathways in the trunk of the chick embryo. Dev. Biol. 1987;121:220–236. - PubMed
-
- Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77:627–638. - PubMed
-
- Brennan C, Rivas-Plata K, Landis SC. The p75 neurotrophin receptor influences NT-3 responsiveness of sympathetic neurons in vivo. Nat. Neurosci. 1999;2:699–705. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

