Structural and ligand recognition characteristics of an acetylcholine-binding protein from Aplysia californica
- PMID: 15069068
- PMCID: PMC4762456
- DOI: 10.1074/jbc.M402452200
Structural and ligand recognition characteristics of an acetylcholine-binding protein from Aplysia californica
Abstract
We generated an acetylcholine-binding protein from Aplysia californica by synthesis of a cDNA found in existing data bases and its expression in mammalian cell culture. Its subunit assembly and ligand recognition behavior were compared with the binding protein previously derived from Lymnaea stagnalis. The secreted proteins were purified by elution from columns of attached antibodies directed to the FLAG epitope encoded in the expression construct. Although the sequences of the two proteins from marine and fresh water mollusks exhibit the characteristic features of the extracellular domain of the nicotinic receptor, they only possess 33% amino acid identity. Both assemble as stable pentamers with five binding sites per pentamer, yet they show distinguishing features of stability and sensitivity to epitope tag placement. Both proteins exhibit changes in tryptophan fluorescence upon ligand binding; however, the magnitude of the changes differs greatly. Moreover, certain ligands show marked differences in dissociation constants for the two proteins and can be regarded as distinguishing or signature ligands. Hence, the two soluble proteins from mollusks, which can be studied by a variety of physical methods, become discrete surrogate proteins for the extracellular domains of distinct subtypes of nicotinic acetylcholine receptors.
Figures


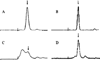
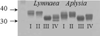
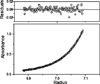
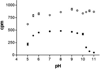


References
-
- Corringer PJ, Le Novere N, Changeux JP. Annu. Rev. Pharmacol. Toxicol. 2000;40:431–458. - PubMed
-
- Karlin A. Nat. Rev. Neurosci. 2002;3:102–114. - PubMed
-
- Sixma TK, Smit AB. Annu. Rev. Biophys. Biomol. Struct. 2003;32:311–334. - PubMed
-
- Chang CC, Lee CY. Arch. Int. Pharmacodyn. Ther. 1963;144:241–257. - PubMed
-
- Lee CY. Showa Igakkai Zasshi. 1963;23:221–229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

