Parallel genetic basis for repeated evolution of armor loss in Alaskan threespine stickleback populations
- PMID: 15069186
- PMCID: PMC395921
- DOI: 10.1073/pnas.0308479101
Parallel genetic basis for repeated evolution of armor loss in Alaskan threespine stickleback populations
Abstract
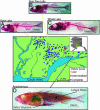
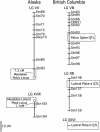
Most adaptation is thought to occur through the fixation of numerous alleles at many different loci. Consequently, the independent evolution of similar phenotypes is predicted to occur through different genetic mechanisms. The genetic basis of adaptation is still largely unknown, however, and it is unclear whether adaptation to new environments utilizes ubiquitous small-effect polygenic variation or large-effect alleles at a small number of loci. To address this question, we examined the genetic basis of bony armor loss in three freshwater populations of Alaskan threespine stickleback, Gasterosteus aculeatus, that evolved from fully armored anadromous populations in the last 14,000 years. Crosses between complete-armor and low-armor populations revealed that a single Mendelian factor governed the formation of all but the most anterior lateral plates, and another independently segregating factor largely determined pelvic armor. Genetic mapping localized the Mendelian genes to different chromosomal regions, and crosses among these same three widely separated populations showed that both bony plates and pelvic armor failed to fully complement, implicating the same Mendelian armor reduction genes. Thus, rapid and repeated armor loss in Alaskan stickleback populations appears to be occurring through the fixation of large-effect variants in the same genes.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

