Mechanisms for activating Cu- and Zn-containing superoxide dismutase in the absence of the CCS Cu chaperone
- PMID: 15069187
- PMCID: PMC395906
- DOI: 10.1073/pnas.0308298101
Mechanisms for activating Cu- and Zn-containing superoxide dismutase in the absence of the CCS Cu chaperone
Erratum in
- Proc Natl Acad Sci U S A. 2004 May 25;101(21):8254
Abstract
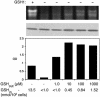
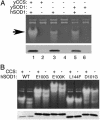
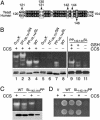
The Cu- and Zn-containing superoxide dismutase 1 (SOD1) largely obtains Cu in vivo by means of the action of the Cu chaperone CCS. Yet, in the case of mammalian SOD1, a secondary pathway of activation is apparent. Specifically, when human SOD1 is expressed in either yeast or mammalian cells that are null for CCS, the SOD1 enzyme retains a certain degree of activity. This CCS-independent activity is evident with both wild-type and mutant variants of SOD1 that have been associated with familial amyotrophic lateral sclerosis. We demonstrate here that the CCS-independent activation of mammalian SOD1 involves glutathione, particularly the reduced form, or GSH. A role for glutathione in CCS-independent activation was seen with human SOD1 molecules that were expressed in either yeast cells or immortalized fibroblasts. Compared with mammalian SOD1, the Saccharomyces cerevisiae enzyme cannot obtain Cu without CCS in vivo, and this total dependence on CCS involves the presence of dual prolines near the C terminus of the SOD1 polypeptide. Indeed, the insertion of such prolines into human SOD1 rendered this molecule refractory to CCS-independent activation. The possible implications of multiple pathways for SOD1 activation are discussed in the context of SOD1 evolutionary biology and familial amyotrophic lateral sclerosis.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

