Tissue factor-factor VIIa-specific up-regulation of IL-8 expression in MDA-MB-231 cells is mediated by PAR-2 and results in increased cell migration
- PMID: 15070680
- PMCID: PMC2837482
- DOI: 10.1182/blood-2003-10-3417
Tissue factor-factor VIIa-specific up-regulation of IL-8 expression in MDA-MB-231 cells is mediated by PAR-2 and results in increased cell migration
Abstract
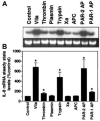
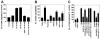
Tissue factor (TF), the cellular receptor for factor VIIa (FVIIa), besides initiating blood coagulation, is believed to play an important role in tissue repair, inflammation, angiogenesis, and tumor metastasis. Like TF, the chemokine interleukin-8 (IL-8) is shown to play a critical role in these processes. To elucidate the potential mechanisms by which TF contributes to tumor invasion and metastasis, we investigated the effect of FVIIa on IL-8 expression and cell migration in a breast carcinoma cell line, MDA-MB-231, a cell line that constitutively expresses abundant TF. Expression of IL-8 mRNA in MDA-MB-231 cells was markedly up-regulated by plasma concentrations of FVII or an equivalent concentration of FVIIa (10 nM). Neither thrombin nor other proteases involved in hemostasis were effective in stimulating IL-8 in these cells. Increased transcriptional activation of the IL-8 gene is responsible for increased expression of IL-8 in FVIIa-treated cells. PAR-2-specific antibodies fully attenuated TF-FVIIa-induced IL-8 expression. Additional in vitro experiments showed that TF-FVIIa promoted tumor cell migration and invasion, active site-inactivated FVIIa, and specific antibodies against TF, PAR-2, and IL-8 inhibited TF-FVIIa-induced cell migration. In summary, the studies described herein provide insight into how TF may contribute to tumor invasion.
Figures
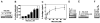
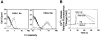



References
-
- Trousseau A. Phlegmasia alba dolens: clinique medicale de l’Hotel-Dieu de Paris. The New Sydenham Society. 1865;3:654–712.
-
- Sack GH, Levin J, Bell WR. Trousseau’s syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: clinical, pathophysiologic, and therapeutic features. Medicine. 1977;56:1–37. - PubMed
-
- Lieberman JS, Borrero J, Urdaneta E, Wright IS. Thrombophlebitis and cancer. JAMA. 1961;177:542–545. - PubMed
-
- Fisher MM, Hochberg LA, Wilensky ND. Recurrent thrombophlebitis on obscure malignant tumors of lung: report of four cases. JAMA. 1951;147:1213–1216. - PubMed
-
- Perlow S, Daniels JL. Venous thrombosis and obscure visceral carcinoma: report of ten cases. Arch Intern Med. 1956;97:184–188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

