Percolation and criticality in a mitochondrial network
- PMID: 15070738
- PMCID: PMC384767
- DOI: 10.1073/pnas.0307156101
Percolation and criticality in a mitochondrial network
Abstract
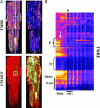
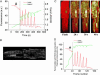
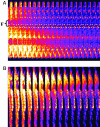
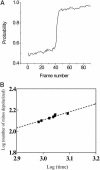
Synchronization of mitochondrial function is an important determinant of cell physiology and survival, yet little is known about the mechanism of interorganellar communication. We have recently observed that coordinated cell-wide oscillations in the mitochondrial energy state of heart cells can be induced by a highly localized perturbation of a few elements of the mitochondrial network, indicating that mitochondria represent a complex, self-organized system. Here, we apply percolation theory to explain the mechanism of intermitochondrial signal propagation in response to oxidative stress. A global phase transition (mitochondrial depolarization) is shown to occur when a critical density of mitochondria accumulate reactive oxygen species above a threshold to form an extended spanning cluster. The scaling and fractal properties of the mitochondrial network at the edge of instability agree remarkably well with the idea that mitochondria are organized as a percolation matrix, with reactive oxygen species as a key messenger.
Figures




References
-
- Aon, M. A. & Cortassa, S. (1997) Dynamic Biological Organization: Fundamentals as Applied to Cellular Systems (Chapman & Hall, London).
-
- Nicolis, G. & Prigogine, I. (1977) Self-Organization in Nonequilibrium Systems: From Dissipative Structures to Order Through Fluctuations (Wiley, New York).
-
- Haken, H. (1991) Synergetics: Can It Help Physiology? (Springer, Berlin).
-
- Kauffman, S. A. (1993) Origins of Order: Self-Organization and Selection in Evolution (Oxford Univ. Press, New York).
-
- Bak, P. (1996) How Nature Works: The Science of Self-Organized Criticality (Copernicus, New York).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

