Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair
- PMID: 15070743
- PMCID: PMC384772
- DOI: 10.1073/pnas.0306068101
Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair
Abstract
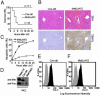
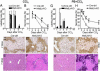
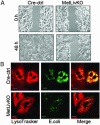
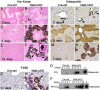
Hepatocyte growth factor/scatter factor c-met signaling pathway is of central importance during development as well as in tumorigenesis. Because homozygous null mice for either hgf/sf or c-met die in utero, we used Cre/loxP-mediated gene targeting to investigate the function of c-met specifically in the adult liver. Loss of c-met appeared not to be detrimental to hepatocyte function under physiological conditions. Nonetheless, the adaptive responses of the liver to injury were dramatically affected. Mice lacking c-met gene in hepatocytes were hypersensitive to Fas-induced apoptosis. When injected with a low dose of anti-Fas antibody, the majority of these mice died from massive apoptosis and hemorrhagic necrosis, whereas all wild-type mice survived with signs of minor injury. After a challenge with a single necrogenic dose of CCl4, c-met conditional knockout mice exhibited impaired recovery from centrolobular lesions rather than a deficit in hepatocyte proliferation. The delayed healing was associated with a persistent inflammatory reaction, over-production of osteopontin, early and prominent dystrophic calcification, and impaired hepatocyte scattering/migration into diseased areas. These studies provide direct genetic evidence in support of the critical role of c-met in efficient liver regeneration and suggest that disruption of c-met affects primarily hepatocyte survival and tissue remodeling.
Figures

References
-
- Michalopoulos, G. K. & DeFrances, M. C. (1997) Science 276, 60–66. - PubMed
-
- Matsumoto, K. & Nakamura, T. (1996) J. Biochem. 119, 591–600. - PubMed
-
- Zhang, Y. W. & Vande Woude, G. F. (2003) J. Cell. Biochem. 88, 408–417. - PubMed
-
- Birchmeier, C. & Gherardi, E. (1998) Trends Cell Biol. 8, 404–410. - PubMed
-
- Vande Woude, G. F., Jeffers, M., Cortner, J., Alvord, G., Tsarfaty, I. & Resau, J. (1997) Ciba Found. Symp. 212, 119–130. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

