Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria
- PMID: 15070757
- PMCID: PMC384786
- DOI: 10.1073/pnas.0400983101
Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria
Abstract
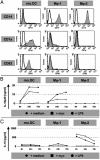
Macrophages (Mphi) play a central role as effector cells in immunity to intracellular pathogens such as Mycobacterium. Paradoxically, they also provide a habitat for intracellular bacterial survival. This paradoxical role of Mphi remains poorly understood. Here we report that this dual role may emanate from the functional plasticity of Mphi: Whereas Mphi-1 polarized in the presence of granulocyte-Mphi colony-stimulating factor promoted type 1 immunity, Mphi-2 polarized with Mphi colony-stimulating factor subverted type 1 immunity and thus may promote immune escape and chronic infection. Importantly, Mphi-1 secreted high levels of IL-23 (p40/p19) but no IL-12 (p40/p35) after (myco)bacterial activation. In contrast, activated Mphi-2 produced neither IL-23 nor IL-12 but predominantly secreted IL-10. Mphi-1 required IFN-gamma as a secondary signal to induce IL-12p35 gene transcription and IL-12 secretion. Activated dendritic cells produced both IL-12 and IL-23, but unlike Mphi-1 they slightly reduced their IL-23 secretion after addition of IFN-gamma. Binding, uptake, and outgrowth of a mycobacterial reporter strain was supported by both Mphi subsets, but more efficiently by Mphi-2 than Mphi-1. Whereas Mphi-1 efficiently stimulated type 1 helper cells, Mphi-2 only poorly supported type 1 helper function. Accordingly, activated Mphi-2 but not Mphi-1 down-modulated their antigen-presenting and costimulatory molecules (HLA-DR, CD86, and CD40). These findings indicate that (i) Mphi-1 and Mphi-2 play opposing roles in cellular immunity and (ii) IL-23 rather than IL-12 is the primary type 1 cytokine produced by activated proinflammatory Mphi-1. Mphi heterogeneity thus may be an important determinant of immunity and disease outcome in intracellular bacterial infection.
Figures




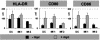
References
-
- Ottenhoff, T. H., Verreck, F. A., Lichtenauer-Kaligis, E. G., Hoeve, M. A., Sanal, O. & Van Dissel, J. T. (2002) Nat. Genet. 32, 97–105. - PubMed
-
- Kaufmann, S. H. (2001) Nat. Rev. Immunol. 1, 20–30. - PubMed
-
- Janeway, C. A., Jr., & Medzhitov, R. (2002) Annu. Rev. Immunol. 20, 197–216. - PubMed
-
- Sieling, P. A. & Modlin, R. L. (2002) Curr. Opin. Microbiol. 5, 70–75. - PubMed
-
- Trinchieri, G. (1995) Annu. Rev. Immunol. 13, 251–276. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

