Differential dependence of CD4+CD25+ regulatory and natural killer-like T cells on signals leading to NF-kappaB activation
- PMID: 15070758
- PMCID: PMC384787
- DOI: 10.1073/pnas.0400885101
Differential dependence of CD4+CD25+ regulatory and natural killer-like T cells on signals leading to NF-kappaB activation
Abstract
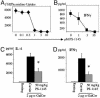
Natural killer-like (NK) T, regulatory T (TR), and memory type T cells display surface phenotypes reminiscent of activated T cells. Previously, we reported that the generation of TR cells and, to a lesser extent, of memory type T cells, depends on IkappaB kinase 2. Here, we show that T cell-specific ablation of IkappaB kinase 2, in addition, completely precludes NKT cell development. T cell antigen receptor (TCR)-induced signals to activate NF-kappaB are essential for mature T cell activation, leading us to hypothesize that this pathway could play an important role in the generation of the antigen-driven T cell subsets comprising TR, memory type T, and NKT cells. TCR-mediated NF-kappaB activation critically depends on Bcl10 and PKCtheta. By using mice deficient for these proteins, we demonstrate that the generation of TR and, to a lesser extent, of memory type T cells, depends on Bcl10 and PKCtheta, and therefore, most likely on NF-kappaB activation initiated by TCR engagement. NKT cells, on the other hand, require PKCtheta for thymic development, whereas absence of Bcl10 leads primarily to the reduction of peripheral NKT cell numbers.
Figures
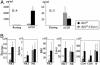



References
-
- Von Boehmer, H., Aifantis, I., Gounari, F., Azogui, O., Haughn, L., Apostolou, I., Jaeckel, E., Grassi, F. & Klein, L. (2003) Immunol. Rev. 191, 62–78. - PubMed
-
- Ramsdell, F. (2003) Immunity 19, 165–168. - PubMed
-
- Kronenberg, M. & Gapin, L. (2002) Nat. Rev. Immunol. 2, 557–568. - PubMed
-
- Shevach, E. M. (2002) Nat. Rev. Immunol. 2, 389–400. - PubMed
-
- Jordan, M. S., Boesteanu, A., Reed, A. J., Petrone, A. L., Holenbeck, A. E., Lerman, M. A., Naji, A. & Caton, A. J. (2001) Nat. Immunol. 2, 301–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

