Mycobacterial polyketide-associated proteins are acyltransferases: proof of principle with Mycobacterium tuberculosis PapA5
- PMID: 15070765
- PMCID: PMC384794
- DOI: 10.1073/pnas.0306928101
Mycobacterial polyketide-associated proteins are acyltransferases: proof of principle with Mycobacterium tuberculosis PapA5
Erratum in
- Proc Natl Acad Sci U S A. 2004 Apr 27;101(17):6834
Abstract
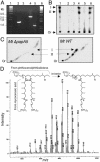
Mycobacterium tuberculosis (Mt) produces complex virulence-enhancing lipids with scaffolds consisting of phthiocerol and phthiodiolone dimycocerosate esters (PDIMs). Sequence analysis suggested that PapA5, a so-called polyketide-associated protein (Pap) encoded in the PDIM synthesis gene cluster, as well as PapA5 homologs found in Mt and other species, are a subfamily of acyltransferases. Studies with recombinant protein confirmed that PapA5 is an acyltransferase [corrected]. Deletion analysis in Mt demonstrated that papA5 is required for PDIM synthesis. We propose that PapA5 catalyzes diesterification of phthiocerol and phthiodiolone with mycocerosate. These studies present the functional characterization of a Pap and permit inferences regarding roles of other Paps in the synthesis of complex lipids, including the antibiotic rifamycin.
Figures



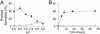
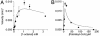

References
-
- Bentley, R. & Bennett, J. W. (1999) Annu. Rev. Microbiol. 53, 411-446. - PubMed
-
- Camacho, L. R., Constant, P., Raynaud, C., Laneelle, M. A., Triccas, J. A., Gicquel, B., Daffe, M. & Guilhot, C. (2001) J. Biol. Chem. 276, 19845-19854. - PubMed
-
- Cox, J. S., Chen, B., McNeil, M. & Jacobs, W. R. (1999) Nature 402, 79-83. - PubMed
-
- Rambukkana, A., Zanazzi, G., Tapinos, N., Salzer, J. L. (2002) Science 296, 927-931. - PubMed
-
- Cole, S. T., Brosch, R., Parkhill, J., Garnier, T., Churcher, C., Harris, D., Gordon, S. V., Eiglmeier, K., Gas, S., Barry, C. E., et al. (1998) Nature 393, 537-544. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

