Salmonella typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway
- PMID: 15070766
- PMCID: PMC384795
- DOI: 10.1073/pnas.0400932101
Salmonella typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway
Abstract
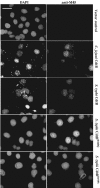
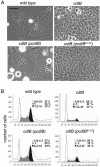
Many bacterial pathogens encode the cytolethal distending toxin (CDT), which causes host cells to arrest during their cell cycle by inflicting DNA damage. CDT is composed of three proteins, CdtA, CdtB, and CdtC. CdtB is the enzymatically active or A subunit, which possesses DNase I-like activity, whereas CdtA and CdtC function as heteromeric B subunits that mediate the delivery of CdtB into host cells. We show here that Salmonella enterica serovar Typhi encodes CDT activity, which depends on the function of a CdtB homologous protein. Remarkably, S. enterica serovar Typhi does not encode apparent homologs of CdtA or CdtC. Instead, we found that toxicity, as well as cdtB expression, requires bacterial internalization into host cells. We propose a pathway of toxin delivery in which bacterial internalization relieves the requirement for the functional equivalent of the B subunit of the CDT toxin.
Figures




References
-
- Johnson, W. M. & Lior, H. (1988) Microb. Pathog. 4, 103-113. - PubMed
-
- Johnson, W. M. & Lior, H. (1988) Microb. Pathog. 4, 115-126. - PubMed
-
- Peres, S., Marches, O., Daigle, F., Nougayrede, J., Herault, F., Tasca, C., De Rycke, J. & Oswald, E. (1997) Mol. Microbiol. 24, 1095-1107. - PubMed
-
- Okuda, J., Kurazono, H. & Takeda, Y. (1995) Microb. Pathog. 18, 167-172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

