Dendritic control of spontaneous bursting in cerebellar Purkinje cells
- PMID: 15071098
- PMCID: PMC6729759
- DOI: 10.1523/JNEUROSCI.0290-04.2004
Dendritic control of spontaneous bursting in cerebellar Purkinje cells
Abstract
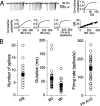
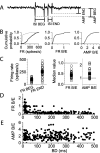
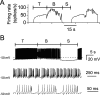
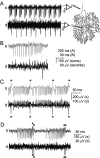
We investigated the mechanisms that contribute to spontaneous regular bursting in adult Purkinje neurons in acutely prepared cerebellar slices. Bursts consisted of 3-20 spikes and showed a stereotypic waveform. Each burst developed with an increase in firing rate and was terminated by a more rapid increase in firing rate and a decrease in spike height. Whole-cell current-clamp recordings showed that each burst ended with a rapid depolarization followed by a hyperpolarization. Dual dendritic and somatic extracellular recordings revealed that each burst was terminated by a dendritic calcium spike. The contributions of T- and P/Q-type calcium current, large (BK) and small (SK) conductance calcium-activated potassium currents, and hyperpolarization-activated (I(H)) current to bursting were investigated with specific channel blockers. None of the currents, except for P/Q, were required to sustain spontaneous bursting or the stereotypic burst waveform. T-type calcium, BK, and SK channels contributed to interspike and interburst intervals. The effect of T-type calcium channel block was more pronounced after BK channel block and vice versa, indicating that these two currents interact to regulate burst firing. Block of I(H) current had no effect on bursting. Partial block of P/Q-type calcium channels concurrently eliminated dendritic calcium spikes and caused a switch from regular bursting to tonic firing or irregular bursting. Dendritic calcium spikes persisted in the presence of tetrodotoxin, indicating that their initiation did not require somatic sodium spikes. Our results demonstrate an important role for dendritic conductances in burst firing in intact Purkinje neurons.
Figures

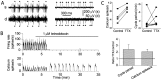

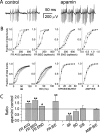

References
-
- Bal T, McCormick DA (1997) Synchronized oscillations in the inferior olive are controlled by the hyperpolarization-activated cation current I(h). J Neurophysiol 77: 3145–3156. - PubMed
-
- Del Negro CA, Hsiao CF, Chandler SH (1999) Outward currents influencing bursting dynamics in guinea pig trigeminal motoneurons. J Neurophysiol 81: 1478–1485. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
