Oligomerization of Alzheimer's beta-amyloid within processes and synapses of cultured neurons and brain
- PMID: 15071107
- PMCID: PMC6729733
- DOI: 10.1523/JNEUROSCI.5167-03.2004
Oligomerization of Alzheimer's beta-amyloid within processes and synapses of cultured neurons and brain
Abstract
Multiple lines of evidence implicate beta-amyloid (Abeta) in the pathogenesis of Alzheimer's disease (AD), but the mechanisms whereby Abeta is involved remain unclear. Addition of Abeta to the extracellular space can be neurotoxic. Intraneuronal Abeta42 accumulation is also associated with neurodegeneration. We reported previously that in Tg2576 amyloid precursor protein mutant transgenic mice, brain Abeta42 localized by immunoelectron microscopy to, and accumulated with aging in, the outer membranes of multivesicular bodies, especially in neuronal processes and synaptic compartments. We now demonstrate that primary neurons from Tg2576 mice recapitulate the in vivo localization and accumulation of Abeta42 with time in culture. Furthermore, we demonstrate that Abeta42 aggregates into oligomers within endosomal vesicles and along microtubules of neuronal processes, both in Tg2576 neurons with time in culture and in Tg2576 and human AD brain. These Abeta42 oligomer accumulations are associated with pathological alterations within processes and synaptic compartments in Tg2576 mouse and human AD brains.
Figures
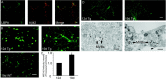
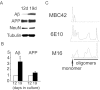
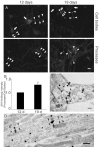


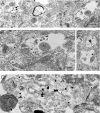
References
-
- Busciglio J, Pelsman A, Wong C, Pigino G, Yuan ML, Mori H, Yankner BA (2002) Altered metabolism of the amyloid beta precursor protein is associated with mitochondrial dysfunction in Down's syndrome. Neuron 33: 677–688. - PubMed
-
- Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, Younkin SG, Hsiao KK (1999) Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci 2: 271–276. - PubMed
-
- D'Andrea MR, Nagele RG, Wang HY, Peterson PA, Lee DH (2001) Evidence that neurones accumulating amyloid can undergo lysis to form amyloid plaques in Alzheimer's disease. Histopathology 38: 120–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
